1,4-Butanediol

Fatal overdose may occur when GABAergic substances are combined with other depressants such as opiates, benzodiazepines, barbiturates, gabapentinoids, thienodiazepines or alcohol.[1]
It is strongly discouraged to combine these substances, particularly in common to heavy doses.
| Summary sheet: 1,4-Butanediol |
| 1,4-Butanediol | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemical Nomenclature | |||||||||||||||||||||||||||||||
| Common names | 1,4-Butanediol, 1,4-B, BD, BDO, One Comma Four, One Four Bee, Butylene Glycol, or One Four B-D-O | ||||||||||||||||||||||||||||||
| Systematic name | Butane-1,4-diol | ||||||||||||||||||||||||||||||
| Class Membership | |||||||||||||||||||||||||||||||
| Psychoactive class | Depressant | ||||||||||||||||||||||||||||||
| Chemical class | Alkanediol | ||||||||||||||||||||||||||||||
| Routes of Administration | |||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
| Interactions | |||||||||||||||||||||||||||||||
| Stimulants | |||||||||||||||||||||||||||||||
| Depressants | |||||||||||||||||||||||||||||||
| Dissociatives | |||||||||||||||||||||||||||||||
1,4-Butanediol (1,4-B, butylene glycol, or BD) is a thick, colourless liquid which is nearly odorless with a distinct bitter taste. 1,4-Butanediol is used industrially as a solvent and in the manufacture of some types of plastics, elastic fibers and polyurethanes. In organic chemistry, 1,4-butanediol is used for the synthesis of γ-butyrolactone (GBL).[3]
In humans, it acts as a depressant and a prodrug for GHB where 1ml is equivalent to 1g of GHB. It is used as a recreational intoxicant with effects similar to alcohol.[4]
1,4-Butanediol, as well as GBL, will dissolve most types of plastic over time.[5] For this reason, it is recommended to only transport and store the drug using a glass container, standard gelatin capsules (not vegetarian), or high-density polyethylene plastic (also known as #2 recycled plastic). To check the type of plastic used on a bottle, one can look at the bottom for a number in the triangle shaped recycling label.

Chemistry
1,4-Butanediol is classified as a subclass of alcoholic compounds called diols. Diols are named for having two alcohol (OH-) substitutions in their structure. 1,4-Butanediol is comprised of a butane chain of four carbon groups with an alcohol group bound to each terminal carbon of this chain. 1,4-Butanediol is named for these alcohol substitutions, which are located at R1 and R4.
Pharmacology
1,4-Butanediol is not active in its own right; its mechanism of action stems from its identity as a prodrug of GHB.[6]
It is converted into GHB in the liver by the enzymes alcohol dehydrogenase and aldehyde dehydrogenase, which is the same enzyme as alcohol.[7] 1,4-Butanediol is first converted into 4-hydroxybutaldehyde in the liver and is released into the bloodstream before returning to the liver to convert into GHB. This process results in a much more delayed onset than GBL or GHB.[8]
The differing levels of dehydrogenase enzymes can vary between individuals, meaning that, like alcohol, effects can differ greatly between users. In many, this manifests as a slow onset of effects and a higher rate of aldehyde collecting in the bloodstream, causing more toxic side effects. Because of these pharmacokinetic differences, 1,4-butanediol tends to be less potent and with a slower onset than GHB but has a longer duration; the related compound GBL tends to be slightly more potent and faster to take effect but more short-acting than GHB.
Subjective effects
Disclaimer: The effects listed below cite the Subjective Effect Index (SEI), an open research literature based on anecdotal user reports and the personal analyses of PsychonautWiki contributors. As a result, they should be viewed with a healthy degree of skepticism.
It is also worth noting that these effects will not necessarily occur in a predictable or reliable manner, although higher doses are more liable to induce the full spectrum of effects. Likewise, adverse effects become increasingly likely with higher doses and may include addiction, severe injury, or death ☠.
Physical effects
- Stimulation and Sedation - At lower doses, 1,4-butanediol is physically stimulating, encouraging movement and wakefulness. At higher doses, however, it becomes physically sedating, encouraging sleep and lethargy.
- Euphoria
- Nausea
- Motor control loss
- Dizziness
- Dehydration
- Respiratory depression - Many reportedly experience an abnormal pattern of breathing characterized by progressively deeper and sometimes faster breathing followed by a gradual decrease that results in a temporary stop in breathing called an apnea.
Cognitive effects
- Empathy, love, and sociability enhancement - Unlike alcohol which merely increases sociability through disinhibition, 1,4-butanediol presents strong entactogenic effects which, although weaker than that of MDMA, are still prominent and well-defined.
- Disinhibition
- Information processing suppression
- Thought deceleration
- Amnesia
- Euphoria
- Anxiety suppression
- Increased libido
- Increased music appreciation
Experience reports
There are currently no anecdotal reports which describe the effects of this compound within our experience index. Additional experience reports can be found here:
Toxicity and harm potential
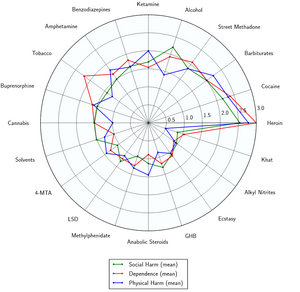
1,4-Butanediol is not active in its own right; its mechanism of action stems from its identity as a prodrug of GHB, meaning that it is rapidly converted into GHB in the body.
GHB is considered to be a safe and non-toxic substance when used responsibly or medically. The LD50 is above the active dosage, and there is no danger of acute toxicity when this compound is taken at appropriate dosages. However, it can be dangerous when used as a recreational drug or abused. There have been many negative reports from recreational users who have overdosed, combined GHB with alcohol or other drugs, or accidentally dosed themselves unexpectedly.[10]
One publication has investigated 226 deaths attributed to GHB.[11] Seventy-one deaths (34%) were caused by GHB alone while the other deaths were from respiratory depression caused by interaction with alcohol or other drugs.
To avoid a possible overdose of GHB/1,4-Butanediol, it is important to start with a low dose and work your way up slowly by increasing the dosage in small increments as the exact toxic dosage is unknown.
Accidental ingestions of 1,4-Butanediol have also occurred due to inadequate storage methods. If 1,4-Butanediol is put into a clear liquid, glass, or bottle, it can be easily mistaken for water. It is recommended to clearly label your 1,4-Butanediol in writing and dye the liquid with blue food coloring so it no longer resembles a drinkable beverage. It is also recommended to store your 1,4-Butanediol in a container that no one would drink out of.
It is strongly recommended that one use harm reduction practices when using this drug.
Neurotoxicity
In multiple studies, GHB has been found to impair spatial memory, working memory, learning and memory in rats with chronic administration.[12][13][14][15] These effects are associated with decreased NMDA receptor expression in the cerebral cortex and possibly other areas as well.[16]
One study found that repeated administration of GHB to rats for 15 days drastically reduced the number of neurons and non-neuronal cells within the hippocampus and in the prefrontal cortex. With doses of 10 mg/kg of GHB, they were decreased by 61% in the hippocampus region and 32% in the prefrontal cortex, and with 100 mg/kg, they were decreased by 38% and 9%, respectively. This paper demonstrates contradicting effects on neuronal loss, with lower doses (10 mg/kg) producing the most neurotoxicity, and higher doses (100 mg/kg) producing less.
Tolerance and addiction potential
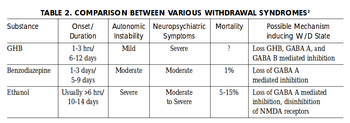
GHB/1,4-Butanediol is moderately physically and psychologically addictive. The frequent use of GHB/1,4-Butanediol can cause withdrawal symptoms similar to those caused by other depressants such as alcohol and benzodiazepines if abruptly discontinued.[18][19] These symptoms seem to depend on the dosage and the length of time the drug was used for. Light to moderate users often experience anxiety, insomnia, sleep-related problems, and tremors whereas heavy use can cause severe withdrawal symptoms like delirium, psychosis, and hallucinations.[20][17]
Although there have been reported fatalities due to GHB/1,4-Butanediol withdrawal, reports are inconclusive and further research is needed.[21]
Tolerance will develop to the sedative-hypnotic effects within several weeks of continuous use. After cessation, the tolerance returns to baseline in 7 - 14 days. Withdrawal symptoms or rebound symptoms may occur after ceasing usage abruptly following a few weeks or longer of steady dosing, and may necessitate a gradual dose reduction.
Dangerous interactions
Warning: Many psychoactive substances that are reasonably safe to use on their own can suddenly become dangerous and even life-threatening when combined with certain other substances. The following list provides some known dangerous interactions (although it is not guaranteed to include all of them).
Always conduct independent research (e.g. Google, DuckDuckGo, PubMed) to ensure that a combination of two or more substances is safe to consume. Some of the listed interactions have been sourced from TripSit.
- Depressants (1,4-Butanediol, 2M2B, alcohol, benzodiazepines, barbiturates, GHB/GBL, methaqualone, opioids) - This combination potentiates the muscle relaxation, amnesia, sedation, and respiratory depression caused by one another. At higher doses, it can lead to a sudden, unexpected loss of consciousness along with a dangerous amount of depressed respiration. There is also an increased risk of suffocating on one's vomit while unconscious. If nausea or vomiting occurs before a loss of consciousness, users should attempt to fall asleep in the recovery position or have a friend move them into it.
- Dissociatives - This combination can unpredictably potentiate the amnesia, sedation, motor control loss and delusions that can be caused by each other. It may also result in a sudden loss of consciousness accompanied by a dangerous degree of respiratory depression. If nausea or vomiting occurs before consciousness is lost, users should attempt to fall asleep in the recovery position or have a friend move them into it.
- Stimulants - Stimulants mask the sedative effect of depressants, which is the main factor most people use to gauge their level of intoxication. Once the stimulant effects wear off, the effects of the depressant will significantly increase, leading to intensified disinhibition, motor control loss, and dangerous black-out states. This combination can also potentially result in severe dehydration if one's fluid intake is not closely monitored. If choosing to combine these substances, one should strictly limit themselves to a pre-set schedule of dosing only a certain amount per hour until a maximum threshold has been reached.
- A review of the details of 194 deaths attributed to or related to GHB over a ten-year period found that most were from respiratory depression caused by interaction with alcohol or other drugs.[22] In humans, GHB has been shown to inhibit the elimination rate of alcohol. This may explain the respiratory arrest that has been reported after ingestion of both drugs.[23] These substances potentiate the muscle relaxation, sedation and amnesia caused by one another and can lead to unexpected loss of consciousness at high doses. There is also an increased risk of vomiting during unconsciousness and death from the resulting suffocation.[24][25] If this occurs, users should attempt to fall asleep in the recovery position or have a friend move them into it.
- Depressants (e.g. 2M2B, alcohol, barbiturates, benzodiazepines, GHB/GBL, methaqualone, opioids) - This combination potentiates the muscle relaxation, amnesia, sedation, and respiratory depression caused by one another and at higher doses, can lead to a sudden, unexpected loss of consciousness along with dangerously depressed respiration. There is also an increased risk of vomiting while unconsciousness and dying from the resulting suffocation. If nausea or vomiting occurs before consciousness is lost, users should attempt to fall asleep in the recovery position or have a friend move them into it.
- Stimulants (e.g. amphetamine, cocaine, methylphenidate, MDMA) - This combination can potentiate the anxiety-inducing, manic, delusional and disinhibiting effects of dissociatives, particularly those without pronounced motor suppressing components such as ketamine. The sum of these effects can increase the likelihood of an anxiety attack, delusions or a psychotic episode. There is also evidence that suggests that combining these two increases their neurotoxicity.[citation needed] Anecdotally, worsened comedowns are also commonly reported when these two classes of substances are combined.
- Dissociatives (e.g. dextromethorphan, ketamine, methoxetamine, PCP) - Combination can unpredictably potentiate amnesia, sedation, motor control loss and delusions. It may also result in a sudden loss of consciousness along with a dangerous amount of respiratory depression. If nausea or vomiting occurs before consciousness is lost, users should attempt to fall asleep in the recovery position or have a friend move them into it.
- Stimulants - It is dangerous to combine 1,4-Butanediol, a depressant, with stimulants due to the risk of excessive intoxication. Stimulants decrease the sedative effect of 1,4-Butanediol, which is the main factor most people consider when determining their level of intoxication. Once the stimulant wears off, the effects of 1,4-Butanediol will be significantly increased, leading to intensified disinhibition as well as other effects. If combined, one should strictly limit themselves to only dosing a certain amount of the drug per hour. This combination can also potentially result in severe dehydration if hydration is not monitored.
Legal issues
- United States: While 1,4-butanediol is not currently scheduled federally in the United States, a number of states have classified it as a controlled substance. Additionally, individuals have been prosecuted for this substance under the Federal Analog Act as being substantially similar to GHB.[26] A federal district court in Chicago ruled that 1,4-butanediol could not be considered an analog of GHB under federal law, and the Seventh Circuit Court of Appeals upheld that ruling.[27]
- United Kingdom: In the United Kingdom, 1,4-butanediol was scheduled in December 2009 (along with another GHB precursor, gamma-butyrolactone) as a Class C controlled substance.
- Germany: In Germany, the drug is not explicitly illegal, but might also be treated as illegal if used as a drug.
- Canada: It is controlled as a Schedule VI precursor in Canada.
See also
External links
References
- ↑ Risks of Combining Depressants - TripSit
- ↑ WHO report on 1,4-butanediol | https://www.who.int/medicines/areas/quality_safety/4_4_Review.pdf
- ↑ http://pubs.acs.org/doi/abs/10.1021/om048983m
- ↑ http://www.nejm.org/doi/full/10.1056/NEJM200101113440202
- ↑ https://www.erowid.org/chemicals/14b/14b_storage1.shtml | 1,4-Butanediol & GBL Storage - 1,4-B & GBL May Dissolve Some Plastics by Erowid (Jul 2001)
- ↑ http://onlinelibrary.wiley.com/doi/10.1111/j.1460-9568.2006.05146.x/abstract;jsessionid=D1EC0DE56C54DB2E93F66DEC2CCB2EDD.f02t02
- ↑ http://emedicine.medscape.com/article/820531-overview | Gamma-Hydroxybutyrate Toxicity
- ↑ http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.htm&r=1&f=G&l=50&s1=8067214.PN.&OS=PN/8067214&RS=PN/8067214
- ↑ Development of a rational scale to assess the harm of drugs of potential misuse (ScienceDirect) | http://www.sciencedirect.com/science/article/pii/S0140673607604644
- ↑ https://www.erowid.org/experiences/exp.php?ID=1926 | Erowid. "GHB Overdoses & Poisonings: An Experience with GHB (ID 1926)". Erowid.org. Jun 19, 2000. erowid.org/exp/1926
- ↑ https://www.ncbi.nlm.nih.gov/pubmed/20825811 | Zvosec DL, Smith SW, Porrata T, Strobl AQ, Dyer JE (2011). "Case series of 226 gamma-hydroxybutyrate-associated deaths: lethal toxicity and trauma". The American Journal of Emergency Medicine 29 (3): 319–32.
- ↑ Adolescent γ-hydroxybutyric acid exposure decreases cortical N-methyl-d-aspartate receptor and impairs spatial learning (ScienceDirect) | http://www.sciencedirect.com/science/article/pii/S009130570400320X
- ↑ Effects of subchronic administration of gammahydroxybutyrate (GHB) on spatial working memory in rats (PubMed.gov / NCBI) | http://www.ncbi.nlm.nih.gov/pubmed/17296081
- ↑ γ-Hydroxybutyric Acid–Induced Cognitive Deficits in the Female Adolescent Rat | http://onlinelibrary.wiley.com/doi/10.1196/annals.1432.044/abstract
- ↑ Neurotoxic effects induced by gammahydroxybutyric acid (GHB) in male rats | http://journals.cambridge.org/action/displayAbstract?fromPage=online&aid=6137924
- ↑ https://www.ncbi.nlm.nih.gov/pubmed/15582677 (PubMed.gov / NCBI) | https://www.ncbi.nlm.nih.gov/pubmed/15582677
- ↑ 17.0 17.1 GHB Withdrawal Syndrome | Texas Commission on Alcohol and Drug Abuse | https://www.erowid.org/chemicals/ghb/ghb_addiction2.pdf
- ↑ Systematic Assessment of Gamma Hydroxybutyrate (GHB) Effects During and After Acute Intoxication (PubMed.gov / NCBI) | https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2759403/
- ↑ Illicit gamma-hydroxybutyrate (GHB) and pharmaceutical sodium oxybate (Xyrem®): differences in characteristics and misuse (PubMed.gov / NCBI) | https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2713368/
- ↑ https://www.ncbi.nlm.nih.gov/pubmed/11174231 | Gamma-hydroxybutyrate withdrawal syndrome.
- ↑ Gamma-hydroxybutyrate: an emerging drug of abuse that causes physical dependence | http://onlinelibrary.wiley.com/doi/10.1111/j.1360-0443.1997.tb03640.x/abstract
- ↑ http://web.archive.org/web/20071203005230/http://www.aafs.org/pdf/Seattleabstracts06.pdf
- ↑ The role of gamma-hydroxybutyric acid in the treatment of alcoholism: from animal to clinical studies (PubMed.gov / NCBI) | http://www.ncbi.nlm.nih.gov/pubmed/10075397
- ↑ https://www.erowid.org/chemicals/ghb/ghb_health.shtml
- ↑ Suspicious death related to gamma-hydroxybutyrate (GHB) toxicity (PubMed.gov / NCBI) | https://www.ncbi.nlm.nih.gov/pubmed/15274975
- ↑ http://cases.justia.com/us-court-of-appeals/F3/312/926/608696/
- ↑ United States v. Turcotte, 405 F.3d 515 (7th Cir. 2005) "With specific regard to 1,4-butanediol, the jury has returned a special verdict which states that 1,4-butanediol is not a Schedule I drug analogue, because 1,4-butanediol's chemical structure is not significantly similar to the chemical structure of GHB.
