Bufotenin
Template:SubstanceBox/5-HO-DMT
 |
This article is a stub. As such, it may contain incomplete or wrong information. You can help by expanding it. |
| Summary sheet: Bufotenin |
Bufotenin (5-HO-DMT, N,N-dimethylserotonin, bufotenine) is a naturally occurring substituted tryptamine alkaloid and a serotonergic psychedelic drug. Bufotenin is a structural derivative of tryptamine and serotonin. Bufotenin is found in a wide array of flora and fauna, including several species of psychoactive toads, most notably the Colorado River toad. The overall effects of bufotenin are generally described as less pleasant than those of other psychedelics such LSD.
Chemistry
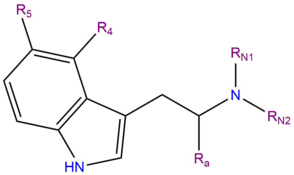
Bufotenin, 5-HO-DMT or 5-hydroxy-N,N-dimethyltryptamine is a ring-substituted indole alkaloid molecule of the tryptamine class. Tryptamines share a core structure comprised of a bicylic indole heterocycle attached at R3 to a terminal amine group via an ethyl side chain. Bufotenin is substituted at R5 of its indole heterocycle with a hydroxy (OH) functional group; it also contains two methyl groups CH3- bound to the terminal amine RN of its tryptamine backbone (DMT).
Pharmacology
Bufotenin's psychedelic effects are primarily believed to come from its efficacy at the 5-HT2A receptor as a partial agonist. Specifically, this molecule shows high binding affinity for the 5-HT2A and 5-HT1A subtypes.[1] However, the role of these interactions and how they result in the psychedelic experience continues to remain elusive.
Additional mechanisms of action such as reuptake inhibition of neurotransmitters such as serotonin, noradrenaline and dopamine are also thought to be involved to an extent.[2] This can result in 5-MeO-DMT becoming dangerously toxic when combined with MAOIs, RIMAs, SSRIs stimulants or any substance which acts as a releasing agent or reuptake inhibitor of monoamine neurotransmitters.
Subjective effects
The effects listed below are based upon the subjective effects index and personal experiences of PsychonautWiki contributors. The listed effects will rarely (if ever) occur all at once, but heavier dosages will increase the chances and are more likely to induce a full range of effects.
Physical effects
The physical effects of Bufotenin can be broken down into several components which progressively intensify proportional to dosage. In comparison to its often relatively mild accompanying cognitive and visual effects, Bufotenin seems to have by far the most proportionally intense and overwhelming physical sensations found within the known psychedelic experience. These individual components are complex, overwhelming and seem to be equally capable of being interpreted as either extremely pleasurable and euphoric or uncomfortable and dysphoric.
- Tactile enhancement - This particular component is perhaps the most overwhelming sensation within the entirety of the Bufotenin experience. It increases the intensity of tactile sensations to such an overwhelming extent that it can induce a sensation of sustained and repeatable full body orgasm within every nerve ending across the entire body to a degree not found within any other psychedelic drug. The experience of this results in the perception of having a difficulty sustaining the act of breathing. It is worth noting, however, that this is not a genuine or dangerous experience of respiratory depression and is considered to be safe.
- Bodily control enhancement
- Bodily pressures
- Changes in gravity
- Euphoria
- Motor control loss
- Nausea
- Perception of increased weight
- Pupil dilation
- Skin flushing
- Temperature regulation suppression
Cognitive effects
- Amnesia
- Analysis enhancement
- Anxiety
- Conceptual thinking
- Delusions
- Subconscious communication
- Emotion enhancement
- Increased music appreciation
- Memory suppression
- Mindfulness
- Thought connectivity
- Time distortion
- Unity and interconnectedness
- Wakefulness
Visual effects
The visual effects of 5-MeO-DMT can be broken down into several components which progressively intensify proportional to dosage. In comparison to its consistently overwhelming and intense accompanying cognitive and physical effects, 5-MeO-DMT seems to have some of the most proportionally underwhelming visual effects found within the known psychedelic experience.
- Visual acuity enhancement and Visual acuity suppression - 5-MeO-DMT is equally capable of both decreasing and increasing visual acuity. The outcome of which effect will manifest seems to be chosen almost entirely at random and is largely setting dependent.
- Drifting (Morphing, Breathing, Melting, Flowing) - In comparison to other psychedelics, this effect can be described as identical to DMT in its style, highly detailed, slow and smooth in motion and static in appearance.
- Colour enhancement
- Colour shifting
- Environmental orbism
- Recursion
The visual geometry that is present throughout this trip does not usually occur and never extends beyond level 7 at its highest state. It is very similar to DMT although significantly smaller in size and more likely to manifest in darkness or without distractions. In terms of appearance, it can be comprehensively described through its variations as intricate in complexity, abstract in form, equally organic and digital in feel, structured in organization, brightly lit, multicoloured in scheme, glossy in shading, equal in sharp and soft edges, small in size, fast in speed, smooth in motion, equal in rounded and angular corners, immersive in depth and consistent in its intensity.
Auditory effects
Natural sources
Colorado River toad


The Colorado River toad (Incilius alvarius), also known as the Sonoran Desert toad, is a psychoactive toad found in northern Mexico and the southwestern United States. Its skin and venom contain 5-MeO-DMT and bufotenin.
The toad's primary defense system are glands that produce a poison that may be potent enough to kill a grown dog.[3] These parotoid glands also produce the 5-MeO-DMT[4] and bufotenin for which the toad is known. Fresh venom can easily be collected from these glands without harm to the toad. To do this, obtain a flat glass plate or any other smooth, nonporous surface of at least 12-inches square and hold the toad in front of the plate (which is fixed in a vertical position). When the desert toad is stroked near the parotid glands in the neck region, there is a squirting out of this venom. When it is allowed to dry on a hard surface it takes on the texture of rubber cement. It contains up to 15% 5-MeO-DMT, as well as N-methyl-5-methoxytryptamine, 5-MeO-NMT and Bufotenine, which have their own entries. In this manner, the venom can be collected on the glass plate, free of dirt and liquid released when the toad is handled.
Dosage
If it is assumed that an average of at least 10% of the venom is 5-MeO-DMT:
- Threshold: 10 - 20 mg
- Light: 20 - 50 mg
- Common: 50 - 100 mg
- Strong: 100 - 200 mg
If it is assumed that an average of at least 25% of the venom is 5-MeO-DMT:
- Threshold: 4 - 8 mg
- Light: 8 - 20 mg
- Common: 20 - 40 mg
- Strong: 40 - 80 mg
Toxicity and harm potential
The toxicity and long-term health effects of recreational 5-MeO-DMT do not seem to have been studied in any scientific context and the exact toxic dose is unknown. This is because 5-MeO-DMT is a research chemical with very little history of human usage. Anecdotal evidence from people within the psychonaut community who have tried 5-MeO-DMT suggests that there are no negative health effects attributed to simply trying the drug by itself at low to moderate doses and using it very sparingly (but nothing can be completely guaranteed). Independent research should always be done to ensure that a combination of two or more substances is safe before consumption.
It is strongly recommended that one use harm reduction practices when using this drug.
Tolerance and addiction potential
5-MeO-DMT is not habit-forming and the desire to use it can actually decrease with use. It is most often self-regulating.
Tolerance to the effects of 5-MeO-DMT are built almost immediately after ingestion. After that, it takes about 1 hour for the tolerance to be reduced to half and 2 hours to be back at baseline (in the absence of further consumption). 5-MeO-DMT does not have a cross-tolerance with other psychedelics, meaning that after the consumption of 5-MeO-DMT psychedelics will not have a reduced effect.
Interactions
Deaths from 5-MeO-DMT are rare but, as a powerful monoamine reuptake inhibitor (MRI), injury can occur when excessive doses are taken or when taken with drugs such as MAOIs, RIMAs, stimulants and any substance which act as a releasing agent or reuptake inhibitor of neurotransmitters such as serotonin and dopamine. This has resulted in well documented deaths[5][6] that are easily avoidable and could have been otherwise prevented.
Legal issues
- Denmark: As of December 1, 2004, 5-MeO-DMT was legally restricted to "medical or scientific purposes".
- Gemany: As of December 1, 2004, 5-MeO-DMT was legally restricted to "medical or scientific purposes".
- Greece: 5-MeO-DMT became a controlled substance in Greece on February 18, 2003 [EU Legal Database].
- Latvia: 5-MeO-DMT is a Schedule I drug.[7]
- New Zealand: 5-MeO-DMT is Schedule I (Class A) in New Zealand.
- Sweden: 5-MeO-DMT was classified as a health hazard under the act "Lagen om förbud mot vissa hälsofarliga varor" (translated as the "Act on the Prohibition of Certain Goods Dangerous to Health") in their regulation SFS 2004:696, making it illegal to sell or possess as of Oct 1, 2004 .[8]
- Switzerland: 5-MeO-DMT is Schedule I in Switzerland.
- Romania: 5-MeO-DMT is illegal in Romania since February 2010.
- USA: 5-MeO-DMT was added to Schedule I, effective January 19, 2011. This means it is illegal to manufacture, buy, possess, or distribute (sell, trade or give) without a DEA license.[9]
- United Kingdom - It is illegal to produce, supply, or import this drug under the Psychoactive Substance Act, which came into effect on May 26th, 2016.[10]
Experience reports
Anecdotal reports which describe this compound within our experience index include:
- Experience: 40mg 5-MeO-DMT (oral) + 40mg MXE (oral) - Untitled
- Experience:A combination of DOC, 5-MAPB, 5-MeO-DMT, ETH-LAD, Cannabis, Pentedrone
Additional experience reports can be found here:
See also
External links
- 5-MeO-DMT (Wikipedia)
- 5-MeO-DMT (Erowid)
- 5-MeO-DMT (TiHKAL)
- 5-MeO-DMT (TripSit)
- 5-MeO-DMT (Bluelight)
- 5-MeO-DMT experiences (Erowid)
References
- ↑ The roles of 5-HT1A and 5-HT2 receptors in the effects of 5-MeO-DMT on locomotor activity and prepulse inhibition in rats (PubMed.gov / NCBI) | http://www.ncbi.nlm.nih.gov/pubmed/17013638
- ↑ The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain (PubMed.gov / NCBI) | http://www.ncbi.nlm.nih.gov/pubmed/17223101
- ↑ Phillips, Steven J. and Wentworth Comus, Patricia, ed. (2000). A Natural History of the Sonoran Desert. University of California Press. p. 537. ISBN 0-520-21980-5.
- ↑ http://www.erowid.org/archive/sonoran_desert_toad/erspamer.htm
- ↑ http://www.ncbi.nlm.nih.gov/pubmed/15214625
- ↑ http://www.ncbi.nlm.nih.gov/pubmed/16356341
- ↑ Noteikumi par Latvijā kontrolējamajām narkotiskajām vielām, psihotropajām vielām un prekursoriem (Triptamīni) | http://likumi.lv/doc.php?id=121086
- ↑ http://www.notisum.se/rnp/sls/sfs/20040696.pdf
- ↑ "DEA_FRDOC_0001-0076".
- ↑ Psychoactive Substances Act 2016 (Legislation.gov.uk) | http://www.legislation.gov.uk/ukpga/2016/2/contents/enacted