DOx
DOx refers to a group of psychedelic amphetamines originally synthesized and studied by Alexander Shulgin in his investigation of psychoactive phenethylamines. Shulgin created most of these substances in the 1970s and later published his findings in his semi-autobiographical book/chemical reference PiHKAL.
The DOx chemicals are highly potent, dose-sensitive, and long-lasting chemicals which are considered more intense and difficult to use than their counterpart 2C-x family. The effects generally consists of strong visuals and an intense body load that is typically accompanied by marked physical and mental stimulation that can persist well past the main duration of its activity (residual stimulation).
Notably, it is one of the few classes of psychedelic chemicals (the other being the 25x-NBOMe series and lysergamides potent enough by weight to be laid and distributed on blotter paper, where it is has a history of being sold as a substitute for real LSD. On the street, they are notorious for their distinctly bitter taste as well as their unusually long come-up period (which can take up to 3 hours before its main effects even begin to present), which has sometimes led LSD users to the premature conclusion that their tabs must be weak or from a "bunk batch" and thus take more to try to compensate, which has led to accidental overdoses and unexpectedly intense experiences when the all the effects finally do take hold.[citation needed]
List of DOx compounds
| Compound | R4 | Structure |
|---|---|---|
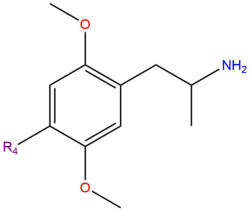
| DOM | CH3 | 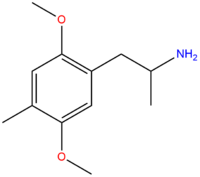
|
| DOET | CH2CH3 | 
|
| DOPR | CH2CH2CH3 | 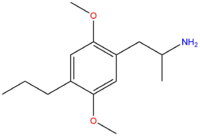
|
| DOiPR | CH(CH3)2 | 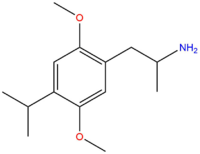
|
| DOBU | (CH2)4CH3 | 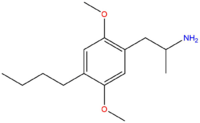
|
| DOAM | (CH2)5CH3 | 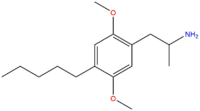
|
| DOF | F | 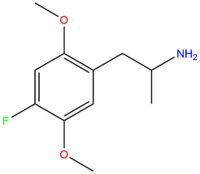
|
| DOC | Cl | 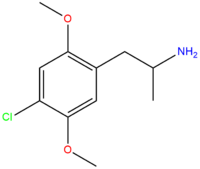
|
| DOB | Br | 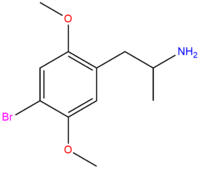
|
| DOI | I | 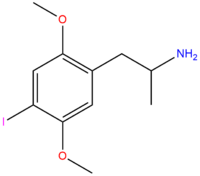
|
| DOEF | CH2CH2F | 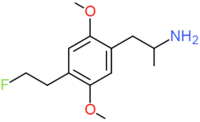
|
| DOTFM | CF3 | 
|
| DON | NO2 | 
|
| TMA-2 | OCH3 | 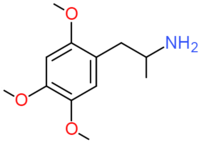
|
| MEM | OCH2CH3 | 
|
| Aleph | SCH3 | 
|
| Aleph-2 | SCH2CH3 | 
|
| Aleph-4 | SCH(CH3)2 | 
|
| Aleph-6 | SC6H5 | 
|
| Aleph-7 | SCH2CH2CH3 | 
|
Health effects, potential addiction and tolerance
 |
This toxicity and harm potential section is a stub. As a result, it may contain incomplete or even dangerously wrong information! You can help by expanding upon or correcting it. |
The DOx chemicals, as with many other serotonergic psychedelics, should not be taken in combination with SSRIs (selective serotonin reuptake inhibitors) or tricyclic antidepressants in general to avoid serotonin syndrome, a potentially life-threatening condition in which an abundance of serotonin builds up past the body's ability to catabolize it, causing many physical and cognitive health problems.
Legal issues
- USA: In the US, some of the DOx chemicals are listed as Schedule I substances and all others under the Federal Analog Act.
- Australia: Australia has a blanket ban over all substituted phenethylamines including the entire DOx family.[1]
- Switzerland: DOM, DOB and DOET are illegal to possess, produce and sell.[2]
See also
References
- ↑ New Psychoactive Substances (National Drug and Alcohol Research Centre 2014) | https://comorbidity.edu.au/sites/default/files/cre/page/New%20Psychoactive%20Substances.pdf
- ↑ https://www.admin.ch/opc/de/classified-compilation/20101220/index.html