25x-NBOMe: Difference between revisions
>BronzeManul m Corrected 'Federal Analog Act' to 'Federal Analogue Act'. |
>BronzeManul Replaced skeletal structure images with standardised svg images. |
||
| Line 22: | Line 22: | ||
! scope="col" | '''Structure''' | ! scope="col" | '''Structure''' | ||
|- | |- | ||
| [[25B-NBOMe]] || Br || [[File: | | [[25B-NBOMe]] || Br || [[File:25B-NBOMe.svg|200px]] | ||
|- | |- | ||
| [[25C-NBOMe]] || Cl || [[File: | | [[25C-NBOMe]] || Cl || [[File:25C-NBOMe.svg|200px]] | ||
|- | |- | ||
| [[25D-NBOMe]] || CH<sub>3</sub> || [[File:25D-NBOMe. | | [[25D-NBOMe]] || CH<sub>3</sub> || [[File:25D-NBOMe.svg|200px]] | ||
|- | |- | ||
| [[25I-NBOMe]] || I || [[File: | | [[25I-NBOMe]] || I || [[File:25I-NBOMe.svg|200px]] | ||
|- | |- | ||
| [[25N-NBOMe]] || NO<sub>2</sub> || [[File: | | [[25N-NBOMe]] || NO<sub>2</sub> || [[File:25N-NBOMe.svg|200px]] | ||
|- | |- | ||
|} | |} | ||
Revision as of 05:31, 30 August 2017

Members of the NBOMe series have been linked to numerous overdoses and fatalities.[1][2][3]
It is strongly discouraged to insufflate (snort) or take higher doses of these compounds. Please see this section for more details.
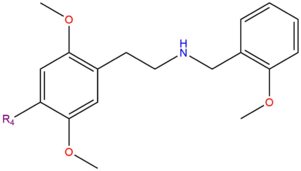
25x-NBOMe is the general form of the NBOMe series of psychedelic phenethylamines. The series had nearly no history of human use before 2010 when some of the first compounds became available for purchase from online research chemical vendors.
While members of this series are often misrepresented as LSD, a fundamental difference between them is that 25x-NBOMe is only active when taken through a sublingual or insufflated route. This means that to get the full effects, 25x-NBOMe blotter paper must be lightly chewed on for 20+ minutes and never immediately swallowed.
25x-NBOMe blotters cause numbness of the tongue and have a distinct taste that is often described as "strongly bitter", "metallic", or "chemical like". This is unlike LSD blotters, which will not cause numbness of the tongue and may be tasteless or have a slight flavor depending on the amount of ink on the blotter and the composition of the blotter paper.
Although these differences can be helpful in identifying an unwanted compound on a blotter, it is recommended that more reliable methods of identification be used, such as the use of testing reagents like the Ehrlich reagent.
Insufflation of these substances are highly discouraged due to numerous reports of people suffering dangerous and often fatal overdoses that have surfaced over the years.[citation needed]
Chemistry
In a comparison of 25x-NBOMe and 2C-x chemicals, each 25x-NBOMe molecule is a serotonergic N-benzyl derivative of the corresponding 2C-x molecule. This change in structure results in an increase in potency. It differs from the related 2C-x structurally through a substitution on the amine (NH2) with a 2-methoxybenzyl (BOMe) group. The addition of this BOMe group also causes most of the compounds to have relatively the same duration, regardless of how long the 2C-x counterpart lasts.
List of 25x-NBOMe compounds
| Compound | R4 | Structure |
|---|---|---|
| 25B-NBOMe | Br | 
|
| 25C-NBOMe | Cl | 
|
| 25D-NBOMe | CH3 | 
|
| 25I-NBOMe | I | 
|
| 25N-NBOMe | NO2 | 
|
Toxicity and harm potential
 |
This toxicity and harm potential section is a stub. As a result, it may contain incomplete or even dangerously wrong information! You can help by expanding upon or correcting it. |
Tolerance and addiction potential
Members of the 25x-NBOMe series are not habit-forming and the desire to use them can actually decrease with use. They are thought to be most often self-regulating.
Tolerance to the effects of any member of the 25x-NBOMe series is built almost immediately after ingestion. After that, it takes about 1 week for the tolerance to be reduced to half and 2 weeks to be back to baseline (in the absence of further consumption). Members of the 25x-NBOMe series present cross-tolerance with [[Cross-tolerance::all psychedelics]], meaning that after the consumption of any member of the 25x-NBOMe series all psychedelics will have a reduced effect.
Dangerous interactions
Warning: Many psychoactive substances that are reasonably safe to use on their own can suddenly become dangerous and even life-threatening when combined with certain other substances. The following list provides some known dangerous interactions (although it is not guaranteed to include all of them).
Always conduct independent research (e.g. Google, DuckDuckGo, PubMed) to ensure that a combination of two or more substances is safe to consume. Some of the listed interactions have been sourced from TripSit.
- Tramadol - Tramadol lowers the seizure threshold[4] and psychedelics may act as triggers for seizures, particularly in those who are predisposed to them.[citation needed]
- Stimulants - Stimulants affect many parts of the brain. Combined with psychedelics, stimulation can turn into uncontrollable anxiety, panic, thought loops and paranoia. This interaction may cause elevated risk of psychosis.[citation needed]
- Lithium - Lithium is often used as treatment for bipolar disorder. It may possibly cause elevated risk of seizures and psychosis due to its glutaminergic and GABAergic effects.[citation needed]
Serotonin syndrome risk
Combinations with the following substances can cause dangerously high serotonin levels. Serotonin syndrome requires immediate medical attention and can be fatal if left untreated.
- MAOIs - Such as banisteriopsis caapi, syrian rue, phenelzine, selegiline, and moclobemide.[5]
- Serotonin releasers - Such as MDMA, 4-FA, methamphetamine, methylone and αMT.
- SSRIs - Such as citalopram and sertraline
- [[Wikipedia:SNRIs|DangerousInteraction::SNRIs]] - Such as tramadol and venlafaxine
- 5-HTP
Legal status
 |
This legality section is a stub. As such, it may contain incomplete or wrong information. You can help by expanding it. |
- United States: In the US, some of the 25x-NBOMe chemicals are listed as Schedule I substances and others may be considered analogues under the Federal Analogue Act.[citation needed]
- United Kingdom - The majority of synthesised 25x-NBOMe substances are Class A drugs in the United Kingdom as a result of the N-benzylphenethylamine catch-all clause.[6] Any compounds not covered by the clause are illegal to produce, supply, or import under the Psychoactive Substance Act, which came into effect on May 26th, 2016.[7]
See also
Literature
- Hansen, M., Phonekeo, K., Paine, J. S., Leth-Petersen, S., Begtrup, M., Bräuner-Osborne, H., & Kristensen, J. L. (2014). Synthesis and structure–activity relationships of N-benzyl phenethylamines as 5-HT2A/2C agonists. ACS Chemical Neuroscience, 5(3), 243-249. https://doi.org/10.1021/cn400216u
- Heim, Ralf (2004). "Synthese und Pharmakologie potenter 5-HT2A-Rezeptoragonisten mit N-2-Methoxybenzyl-Partialstruktur". Freie Universität Berlin. Retrieved 27 June 2015.
- Hansen, M.; Phonekeo, K.; Paine, J. S.; Leth-Petersen, S.; Begtrup, M.; Bräuner-Osborne, H.; Kristensen, J. L. (2014). "Synthesis and Structure-Activity Relationships of N-Benzyl Phenethylamines as 5-HT2A/2C Agonists". ACS Chemical Neuroscience. 5 (3): 243–9. PMC 3963123 Freely accessible. PMID 24397362. https://doi.org/10.1021/cn400216u
- Ettrup, A.; Hansen, M.; Santini, M. A.; Paine, J.; Gillings, N.; Palner, M.; Lehel, S.; Herth, M. M.; Madsen, J. (2010). "Radiosynthesis and in vivo evaluation of a series of substituted 11C-phenethylamines as 5-HT2A agonist PET tracers". European Journal of Nuclear Medicine and Molecular Imaging. 38 (4): 681–693. PMID 21174090. https://doi.org/10.1007/s00259-010-1686-8
References
- ↑ Erowid 25I-NBOMe (2C-I-NBOMe) Vault : Fatalities / Deaths
- ↑ Erowid 2C-C-NBOMe (25C-NBOMe) Vault : Fatalities / Deaths
- ↑ Erowid NBOMe (Other or Unknown NBOMe-Compound) Vault : Fatalities / Deaths
- ↑ Talaie, H., Panahandeh, R., Fayaznouri, M. R., Asadi, Z., & Abdollahi, M. (2009). Dose-independent occurrence of seizure with tramadol. Journal of medical toxicology, 5(2), 63-67. doi:10.1007/BF03161089
- ↑ Gillman, P. K. (2005). "Monoamine oxidase inhibitors, opioid analgesics and serotonin toxicity". British Journal of Anaesthesia. 95 (4): 434–441. doi:10.1093/bja/aei210
. eISSN 1471-6771. ISSN 0007-0912. OCLC 01537271. PMID 16051647.
- ↑ United Kingdom. (2014). Misuse of Drugs Act 1971 (S.I. 2014/1106). London: The Stationery Office Limited. Retrieved July 5, 2017, from http://www.legislation.gov.uk/uksi/2014/1106/made
- ↑ Psychoactive Substances Act 2016 (Legislation.gov.uk) | http://www.legislation.gov.uk/ukpga/2016/2/contents/enacted