3-FEA: Difference between revisions
>TheBaggyMan m Grammatics |
>BronzeManul m Corrected 'Federal Analog Act' to 'Federal Analogue Act', did some minor rewording of the United States legality statement, and corrected 'analogue' to 'analog' in UK legality statement. |
||
| Line 113: | Line 113: | ||
{{legalStub}} | {{legalStub}} | ||
3-FEA is currently a grey area compound within all parts of the world, meaning its regulation lies in a legal grey area and that it is not known to be specifically illegal ("scheduled") within any country. However, individuals may still be charged for its possession under certain circumstances such as under analogue laws and with intent to sell or consume. | 3-FEA is currently a grey area compound within all parts of the world, meaning its regulation lies in a legal grey area and that it is not known to be specifically illegal ("scheduled") within any country. However, individuals may still be charged for its possession under certain circumstances such as under analogue laws and with intent to sell or consume. | ||
*'''United States:''' 3-FEA may be considered to be an | *'''United States:''' 3-FEA may be considered to be an analogue of amphetamine under the Federal Analogue Act and thus a Schedule II drug. The Federal Analogue Act, 21 U.S.C. § 813, is a section of the United States Controlled Substances Act, allowing any chemical "substantially similar" to an illegal drug (in Schedule I or II) to be treated as if it were also in Schedule I or II, but only if it is intended for human consumption. | ||
*'''United Kingdom:''' 3-FEA is considered a Class A drug as a result of the amphetamine | *'''United Kingdom:''' 3-FEA is considered a Class A drug as a result of the amphetamine analog clause of the Misuse of Drugs Act 1971.<ref>Misuse of Drugs Act 1971 (Legislation.gov.uk) |http://www.legislation.gov.uk/ukpga/1971/38/schedule/2/part/I</ref> | ||
*'''Canada:''' 3-FEA would be considered Schedule I as it is an analogue of Amphetamine.<ref>Controlled Drugs and Substances Act (S.C. 1996, c. 19) |http://laws-lois.justice.gc.ca/eng/acts/C-38.8/page-12.html#h-28</ref> | *'''Canada:''' 3-FEA would be considered Schedule I as it is an analogue of Amphetamine.<ref>Controlled Drugs and Substances Act (S.C. 1996, c. 19) |http://laws-lois.justice.gc.ca/eng/acts/C-38.8/page-12.html#h-28</ref> | ||
*'''New Zealand:''' 3-FEA is an amphetamine analogue, so is a Schedule 3 controlled substance in New Zealand.<ref>http://www.legislation.govt.nz/act/public/1975/0116/latest/whole.html#DLM436576</ref> | *'''New Zealand:''' 3-FEA is an amphetamine analogue, so is a Schedule 3 controlled substance in New Zealand.<ref>http://www.legislation.govt.nz/act/public/1975/0116/latest/whole.html#DLM436576</ref> | ||
Revision as of 00:10, 25 August 2017
This page has not been fully approved by the PsychonautWiki administrators. It may contain incorrect information, particularly with respect to dosage, duration, subjective effects, toxicity and other risks. It may also not meet PW style and grammar standards. |
| Summary sheet: 3-FEA |
| 3-FEA | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Nomenclature | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common names | 3-FEA | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substitutive name | 3-Fluoroethamphetamine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Systematic name | N-Ethyl-1-(3-fluorophenyl)propan-2-amine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class Membership | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Psychoactive class | Entactogen / Stimulant | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical class | Amphetamine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Routes of Administration | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Interactions | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alcohol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GHB | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GBL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Opioids | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cannabis | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Caffeine | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ketamine | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Methoxetamine | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Psychedelics | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cocaine | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DXM | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PCP | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 25x-NBOMe | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2C-T-x | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5-MeO-xxT | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| DOx | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tramadol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| aMT | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MAOIs | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
3-Fluoroethamphetamine (also known as 3-FEA) is a novel synthetic ring-substituted fluorinated amphetamine compound that produces a mixture of entactogenic and stimulant effects when administered. 3-FEA is structurally related to a series of designer fluorinated substituted amphetamines that originally included compounds such as 2-FA, 2-FMA, 3-FA, 4-FMA, 4-FA.[1]
Like its parent compound 3-FA, the pharmacological, toxicological, and subjective effects of 3-FEA in humans have yet to be mapped out in detail. Early reports have characterized 3-FEA as a moderately potent serotonin and norepinephrine-dominant triple monoamine releaser that produces a medium-duration mixture of entactogenic and stimulating effects that skew more to the euphoric-recreational side (similar to one of its popular predecessors, 4-FA), than towards what some consider to be "functional" or "productivity-enhancing" (e.g. isopropylphenidate, 2-FA, or 2-FMA).
It should be noted that based on the pharmacology activity displayed by structurally similar compounds such as fenfluramine, it is possible that this compound may possess notable neurotoxic, cardiotoxic and other potentially undiscovered toxic properties.[citation needed]
3-FEA has an extremely short history of human recreational use and has yet to be documented being sold on the streets. It has recently been made available for sale on the gray market as a research chemical by online vendors.[citation needed] Due to its potent psychostimulant effects, likely habit-forming properties as well as unknown toxicity profile, it is strongly recommended that one use proper harm reduction practices if choosing to use this substance.
Chemistry
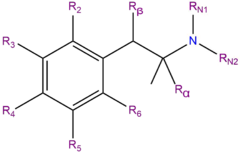
3-FEA, or 3-fluoroethamphetamine, is a synthetic molecule of the amphetamine chemical class. Molecules of the amphetamine class contain a phenethylamine core comprised of a phenyl ring bound to an amino (NH2) group through an ethyl chain substituted with a methyl group at Rα (i.e. amphetamines are alpha-methylated phenethylamines).
3-FEA is the 3-position fluorinated analog of ethylamphetamine (also known as ethamphetamine). It is also an analog of fenfluramine with the 3-trifluoromethyl group replaced with a 3-fluoro substituent.[citation needed]
Pharmacology
Although 3-FEA has not been formally studied on the same level as traditional amphetamines, it is currently assumed that like other substituted amphetamines with substitutions at similar positions, it most likely acts primarily as a triple reuptake inhibitor and/or releaser of the monoamine neurotransmitters serotonin, dopamine, and norepinephrine.[2][3]
It has been demonstrated that compared to the unsubstituted ethylamphetamine, 3-fluoroethamphetamine is a weaker releaser of dopamine, but a stronger releaser of both noradrenaline and serotonin, producing the strongest reinforcing effects in animal studies out of a range of 3-substituted amphetamine derivatives tested, despite not being the most potent dopamine releaser.[3][2]
This indicates that 3-FEA effectively increases the levels of all the three major monoamine neurotransmitters dopamine, norepinephrine, and serotonin in the brain by binding to and partially blocking the transporter proteins that normally clear those molecules from the synaptic cleft after they have fulfilled their function of conducting a neural impulse. This transporter blockade allows these molecules to accumulate within core synaptic regions of the brain to extra-endogenous levels, resulting in a combination of relaxing, stimulating, disinhibiting and euphoric effects associated with entactogenic substituted amphetamines such as MDMA or 4-FA.[citation needed]
Subjective effects
 |
This subjective effects section is a stub. As such, it is still in progress and may contain incomplete or wrong information. You can help by expanding or correcting it. |
Unlike its analogs 2-FA and 2-FMA, both of which have been reported as being relatively functional and non-recreational stimulants due to the "dose-ceilings" that limit the euphoria they can produce, it is likely 3-FEA has more abuse potential and the ability to induce compulsive redosing due to its mix of entactogenic and stimulant effects. Early reports suggest it is a poor candidate for functional use and is typically better suited for occasional recreational outings, and only used sparingly and cautiously.
Disclaimer: The effects listed below cite the Subjective Effect Index (SEI), an open research literature based on anecdotal user reports and the personal analyses of PsychonautWiki contributors. As a result, they should be viewed with a healthy degree of skepticism.
It is also worth noting that these effects will not necessarily occur in a predictable or reliable manner, although higher doses are more liable to induce the full spectrum of effects. Likewise, adverse effects become increasingly likely with higher doses and may include addiction, severe injury, or death ☠.
Physical effects
- Spontaneous physical sensations - Early reports have described the "body high" of 3-FEA as a moderate to strong euphoric tingling sensation that can encompass the entire body that is capable of becoming extremely pleasurable at higher dosages. This sensation maintains a consistent presence that steadily rises with the onset and hits its limit once the peak has been reached.
- Tactile enhancement - This component primarily tends to occur with higher doses.
- Stimulation & Sedation - 3-FEA has been reported to produce a paradoxical combination of stimulating and sedating effects, with the sedating aspects believed to be attributable to the significant amount of serotonin it releases. However, this aspect is not as pronounced as it is with heavier serotonin-dominant releasing agents such MDMA, MDA or MDEA.
- Perception of bodily heaviness - Depending on whether one is feeling more stimulated than sedated, one will often feel as if they are lighter (as in this case) or heavier in the other.
- Abnormal heartbeat[citation needed]
- Increased heart rate[citation needed]
- Increased blood pressure[citation needed]
- Increased perspiration
- Temperature regulation suppression[citation needed]
- Headaches
- Vasoconstriction[citation needed]
- Dehydration
- Dry mouth[citation needed]
- Difficulty urinating - Higher doses of 3-FEA result in an overall difficulty when it comes to urination, an effect that is usually temporary and thought to be harmless.
- Appetite suppression
- Pupil dilation
- Teeth grinding - This component can be considered to be less intense when compared with that of MDMA as well as 2-FMA.
Cognitive effects
- Anxiety suppression or Anxiety - While common doses have been reported to typically produce anxiety suppressing effects characteristic of serotonin-releasing entactogens, higher doses of 3-FEA have been reported to produce states of anxiety, particularly during the come up and offset phases.
- Disinhibition
- Empathy, affection, and sociability enhancement
- Ego inflation
- Thought acceleration
- Cognitive euphoria
- Increased music appreciation
- Increased libido
- Immersion enhancement
- Motivation enhancement
- Compulsive redosing
- Time distortion - This can be described as the experience of time speeding up and passing much quicker than it usually would when sober.
- Wakefulness
After effects
The effects which occur during the offset of a stimulant experience generally feel negative and uncomfortable in comparison to the effects which occurred during its peak. This is often referred to as a "comedown" and occurs because of neurotransmitter depletion. Its effects commonly include:
- Anxiety
- Cognitive fatigue
- Depression
- Irritability
- Motivation suppression
- Thought deceleration
- Wakefulness
Experience reports
Anecdotal reports which describe the effects of this compound within our experience index include:
Additional experience reports can be found here:
Toxicity and harm potential
The toxicity and long-term health effects of recreational 3-FEA use do not seem to have been studied in any scientific context and the exact toxic dosage is unknown. This is because 3-FEA has an extremely brief history of human usage, first becoming available in mid-2016. Early anecdotal reports from people within the community who have tried 3-FEA suggest that there do not seem to be any negative health effects attributed to simply trying this substance at low to moderate doses by itself and using it very cautiously sparingly (although nothing can be completely guaranteed).
Potential cardiotoxicity
Due to its serotonin-releasing entactogenic properties, it is possible 3-FEA may display significant affinity and activity at the 5-HT2B receptor, which like 5-HT2B agonists such as MDMA and fenfluramine would make it cardiotoxic with long-term or heavy use.[4]
It is strongly recommended that one use proper harm reduction practices when using this substance.
Tolerance and addiction potential
Although it still remains to be seen, the chronic use of 3-FEA will likely come to be considered to be moderately addictive with a high potential for abuse and capable of causing psychological dependence among a certain population of users. When dependence or addiction has developed, cravings and withdrawal effects may occur if a person suddenly stops their usage.
Tolerance to many of the effects of 3-FEA develops with prolonged and repeated use. This results in users having to administer increasingly large doses to achieve the same effects. Afterward, it takes about 2 - 3 days for the tolerance to be reduced to half and 3-7 days to be back at baseline (in the absence of further consumption). 3-FEA likely presents cross-tolerance with [[Cross-tolerance::all dopaminergic and serotonergic stimulants]] and entactogens, meaning that after the consumption of 3-FEA all stimulants will have a reduced effect (including atypical stimulants one might not expect, such MDMA or amphetamine due to its reliance on robust dopamine and norepinephrine stores to exert its full spectrum of effect).
Psychosis
Abuse of compounds within the amphetamine chemical class at high dosages for prolonged periods of time can potentially result in a stimulant psychosis that may present with a variety of symptoms (e.g., paranoia, hallucinations, or delusions).[5] A review on treatment for amphetamine, dextroamphetamine, and methamphetamine abuse-induced psychosis states that about 5–15% of users fail to recover completely.[6][7] The same review asserts that, based upon at least one trial, antipsychotic medications effectively resolve the symptoms of acute amphetamine psychosis.[8] Psychosis very rarely arises from therapeutic use.[9][10]
Dangerous interactions
Warning: Many psychoactive substances that are reasonably safe to use on their own can suddenly become dangerous and even life-threatening when combined with certain other substances. The following list provides some known dangerous interactions (although it is not guaranteed to include all of them).
Always conduct independent research (e.g. Google, DuckDuckGo, PubMed) to ensure that a combination of two or more substances is safe to consume. Some of the listed interactions have been sourced from TripSit.
- "[[DangerousInteraction" contains a listed "[" character as part of the property label and has therefore been classified as invalid.]] - This combination may increase the amount of neurotransmitters such as dopamine to dangerous or even fatal levels. Examples include syrian rue, banisteriopsis caapi, and some antidepressants.[11]
- Stimulants - 3-FEA can be potentially dangerous in combination with other stimulants as it increase one's heart rate and blood pressure to dangerous levels.
- "[[DangerousInteraction" contains a listed "[" character as part of the property label and has therefore been classified as invalid.]] & "[[DangerousInteraction" contains a listed "[" character as part of the property label and has therefore been classified as invalid.]] - 25x compounds are highly stimulating and physically straining. Combinations with 3-FEA should be strictly avoided due to the risk of excessive stimulation and heart strain. This can result in increased blood pressure, vasoconstriction, panic attacks, thought loops, seizures, and heart failure in extreme cases.
- "[[UncertainInteraction" contains a listed "[" character as part of the property label and has therefore been classified as invalid.]] - Combining alcohol with stimulants can be dangerous due to the risk of accidental over-intoxication. Stimulants mask alcohol's depressant effects, which is what most people use to assess their degree of intoxication. Once the stimulant wears off, the depressant effects will be left unopposed, which can result in blackouts and severe respiratory depression. If mixing, the user should strictly limit themselves to only drinking a certain amount of alcohol per hour.
- "[[UnsafeInteraction" contains a listed "[" character as part of the property label and has therefore been classified as invalid.]] - Combinations with DXM should be avoided due to its inhibiting effects on serotonin and norepinephrine reuptake. There is an increased risk of panic attacks and hypertensive crisis, or serotonin syndrome with serotonin releasers (MDMA, methylone, mephedrone, etc.). Monitor blood pressure carefully and avoid strenuous physical activity.
- "[[UnsafeInteraction" contains a listed "[" character as part of the property label and has therefore been classified as invalid.]] - Any neurotoxic effects of MDMA are likely to be increased when other stimulants are present. There is also a risk of excessive blood pressure and heart strain (cardiotoxicity).
- "[[UncertainInteraction" contains a listed "[" character as part of the property label and has therefore been classified as invalid.]] - Some reports suggest combinations with MXE may dangerously increase blood pressure and increase the risk of mania and psychosis.
- "[[UncertainInteraction" contains a listed "[" character as part of the property label and has therefore been classified as invalid.]] - Both classes carry a risk of delusions, mania and psychosis, and these risk may be multiplied when combined.
- "[[UnsafeInteraction" contains a listed "[" character as part of the property label and has therefore been classified as invalid.]] - 3-FEA may be dangerous to combine with other stimulants like cocaine as they can increase one's heart rate and blood pressure to dangerous levels.
- "[[DangerousInteraction" contains a listed "[" character as part of the property label and has therefore been classified as invalid.]] - Tramadol is known to lower the seizure threshold[12] and combinations with stimulants may further increase this risk.
- MDMA - The neurotoxic effects of MDMA may be increased when combined with other amphetamines or monoamine releasing agents.
- Cocaine - This combination may increase strain on the heart to dangerous levels.
Serotonin syndrome risk
Combinations with the following substances can cause dangerously high serotonin levels. Serotonin syndrome requires immediate medical attention and can be fatal if left untreated.
- MAOIs - Such as banisteriopsis caapi, syrian rue, phenelzine, selegiline, and moclobemide.[13]
- Serotonin releasers - Such as MDMA, 4-FA, methamphetamine, methylone and αMT.
- SSRIs - Such as citalopram and sertraline
- [[Wikipedia:SNRIs|DangerousInteraction::SNRIs]] - Such as tramadol and venlafaxine
- 5-HTP
Legality
 |
This legality section is a stub. As such, it may contain incomplete or wrong information. You can help by expanding it. |
3-FEA is currently a grey area compound within all parts of the world, meaning its regulation lies in a legal grey area and that it is not known to be specifically illegal ("scheduled") within any country. However, individuals may still be charged for its possession under certain circumstances such as under analogue laws and with intent to sell or consume.
- United States: 3-FEA may be considered to be an analogue of amphetamine under the Federal Analogue Act and thus a Schedule II drug. The Federal Analogue Act, 21 U.S.C. § 813, is a section of the United States Controlled Substances Act, allowing any chemical "substantially similar" to an illegal drug (in Schedule I or II) to be treated as if it were also in Schedule I or II, but only if it is intended for human consumption.
- United Kingdom: 3-FEA is considered a Class A drug as a result of the amphetamine analog clause of the Misuse of Drugs Act 1971.[14]
- Canada: 3-FEA would be considered Schedule I as it is an analogue of Amphetamine.[15]
- New Zealand: 3-FEA is an amphetamine analogue, so is a Schedule 3 controlled substance in New Zealand.[16]
See also
External links
References
- ↑ Quednow, B., Girreser, U., Junge, T., & Ro, P. (2005). Isomeric Fluoro-methoxy-phenylalkylamines: a new series of controlled-substance analogues (designer drugs), 148, 143–156. https://doi.org/10.1016/j.forsciint.2004.05.003
- ↑ 2.0 2.1 Tessel RE, Rutledge CO. Specificity of release of biogenic amines from isolated rat brain tissue as a function of the meta substituent of N-ethylamphetamine derivatives. The Journal of Pharmacology and Experimental Therapeutics 1976;15:142.
- ↑ 3.0 3.1 Tessel RE, Woods JH. Substituted N-ethylamphetamine self injection responding in the rhesus monkey: structure-activity relationships. The Journal of Pharmacology and Experimental Therapeutics 1978; 2: 274–81.
- ↑ Huang, X. P., Setola, V., Yadav, P. N., Allen, J. A., Rogan, S. C., Hanson, B. J., ... & Roth, B. L. (2009). Parallel functional activity profiling reveals valvulopathogens are potent 5-hydroxytryptamine2B receptor agonists: implications for drug safety assessment. Molecular Pharmacology, 76(4), 710-722. https://doi.org/10.1161/01.CIR.102.23.2836
- ↑ Treatment for amphetamine psychosis | [1]
- ↑ Treatment for amphetamine psychosis | [2]
- ↑ Hofmann FG (1983). A Handbook on Drug and Alcohol Abuse: The Biomedical Aspects (2nd ed.). New York: Oxford University Press. p. 329. ISBN 9780195030570.
- ↑ Treatment for amphetamine psychosis | [3]
- ↑ Stimulant Misuse: Strategies to Manage a Growing Problem | http://www.acha.org/prof_dev/ADHD_docs/ADHD_PDprogram_Article2.pdf
- ↑ http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021303s026lbl.pdf
- ↑ Gillman, P. K. (2005). "Monoamine oxidase inhibitors, opioid analgesics and serotonin toxicity". British Journal of Anaesthesia. 95 (4): 434–441. doi:10.1093/bja/aei210
. eISSN 1471-6771. ISSN 0007-0912. OCLC 01537271. PMID 16051647.
- ↑ Talaie, H.; Panahandeh, R.; Fayaznouri, M. R.; Asadi, Z.; Abdollahi, M. (2009). "Dose-independent occurrence of seizure with tramadol". Journal of Medical Toxicology. 5 (2): 63–67. doi:10.1007/BF03161089. eISSN 1937-6995. ISSN 1556-9039. OCLC 163567183.
- ↑ Gillman, P. K. (2005). "Monoamine oxidase inhibitors, opioid analgesics and serotonin toxicity". British Journal of Anaesthesia. 95 (4): 434–441. doi:10.1093/bja/aei210
. eISSN 1471-6771. ISSN 0007-0912. OCLC 01537271. PMID 16051647.
- ↑ Misuse of Drugs Act 1971 (Legislation.gov.uk) |http://www.legislation.gov.uk/ukpga/1971/38/schedule/2/part/I
- ↑ Controlled Drugs and Substances Act (S.C. 1996, c. 19) |http://laws-lois.justice.gc.ca/eng/acts/C-38.8/page-12.html#h-28
- ↑ http://www.legislation.govt.nz/act/public/1975/0116/latest/whole.html#DLM436576