This is an unofficial archive of PsychonautWiki as of 2025-08-11T15:14:44Z. Content on this page may be outdated, incomplete, or inaccurate. Please refer to the original page for the most up-to-date information.
Substituted benzofurans: Difference between revisions
Jump to navigation
Jump to search
>Dextromethorphan |
>Oskykins No edit summary |
||
| Line 1: | Line 1: | ||
{{stub}} | {{stub}} | ||
[[File:Benzofuran.png|170px|thumbnail|The benzofuran molecule]] | [[File:Benzofuran.png|170px|thumbnail|The benzofuran molecule]] | ||
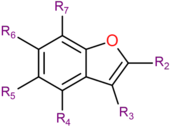
[[File:SBenzofuran.png|thumb|right|170px||General formula of a benzofuran class molecule | [[File:SBenzofuran.png|thumb|right|170px||General formula of a benzofuran class molecule]] | ||
'''Benzofurans''' are chemical compounds | '''Benzofurans''' are chemical compounds which contain a benzofuran ring in their structure. Most of the benzofurans are [[research chemicals]], most commonly [[stimulants]] and [[entactogens]], and are fairly new. Benzofuran compounds are often analogues of [[MDxx]] and [[tryptamine]] compounds where a benzofuran ring has replaced the 3,4-methylenedioxy benzene ring structure or indole ring respectively. | ||
== List of Benzofuran compounds == | == List of Benzofuran compounds == | ||
Revision as of 01:04, 30 October 2016
 |
This article is a stub. As such, it may contain incomplete or wrong information. You can help by expanding it. |
Benzofurans are chemical compounds which contain a benzofuran ring in their structure. Most of the benzofurans are research chemicals, most commonly stimulants and entactogens, and are fairly new. Benzofuran compounds are often analogues of MDxx and tryptamine compounds where a benzofuran ring has replaced the 3,4-methylenedioxy benzene ring structure or indole ring respectively.
List of Benzofuran compounds
| Compound | R2 | R3 | R4 | R5 | R6 | R7 | Structure |
|---|---|---|---|---|---|---|---|
| 2-APB | CH2CH(NH2)CH3 | H | H | H | H | H | 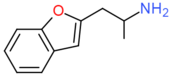
|
| 2-MAPB | CH2CH(NHCH3)CH3 | H | H | H | H | H | 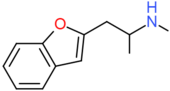
|
| 2-EAPB | CH2CH(NHCH2CH3)CH3 | H | H | H | H | H | 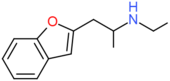
|
| 3-APB | H | CH2CH(NH2)CH3 | H | H | H | H | 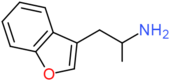
|
| Mebfap | H | CH2CH(NH2)CH3 | H | OCH3 | H | H | 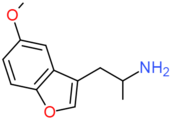
|
| 5-MeO-BFE | H | CH2CH2N(CH3)2 | H | OCH3 | H | H | 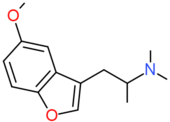
|
| 5-MeO-DiBF | H | CH2CH2N(CH(CH3)2)2 | H | OCH3 | H | H | 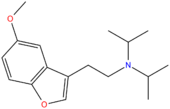
|
| 4-APB | H | H | CH2CH(NH2)CH3 | H | H | H | 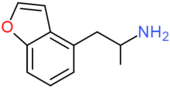
|
| 5-MeO-7-Br-4-APDB | H2 | H2 | CH2CH(NH2)CH3 | OCH3 | H | Br | 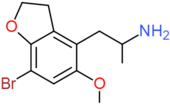
|
| 5-APB | H | H | H | CH2CH(NH2)CH3 | H | H | 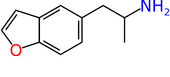
|
| 5-APDB | H2 | H2 | H | CH2CH(NH2)CH3 | H | H | 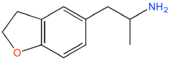
|
| 5-MAPB | H | H | H | CH2CH(NHCH3)CH3 | H | H | 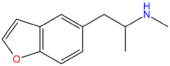
|
| 5-MAPDB | H2 | H2 | H | CH2CH(NHCH3)CH3 | H | H | 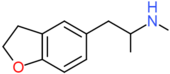
|
| bk-5-MAPB | H | H | H | COCH(NHCH3)CH3 | H | H | 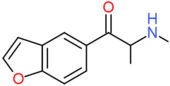
|
| 5-EAPB | H | H | H | CH2CH(NHCH2CH3)CH3 | H | H | 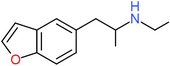
|
| 5-EAPDB | H2 | H2 | H | CH2CH(NHCH2CH3)CH3 | H | H | 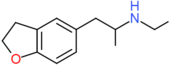
|
| 5-MBPB | H | H | H | CH2CH(NHCH3)CH2CH3 | H | H | 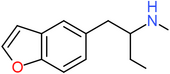
|
| 5-DBFPV | H2 | H2 | H | COCH(NC4H8)CH2CH2CH3 | H | H | 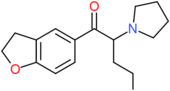
|
| 6-MeO-5-APDB | H2 | H2 | H | CH2CH(NH2)CH3 | OCH3 | H | 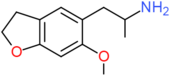
|
| 6-APB | H | H | H | H | CH2CH(NH2)CH3 | H | 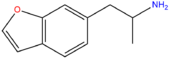
|
| 6-APDB | H2 | H2 | H | H | CH2CH(NH2)CH3 | H | 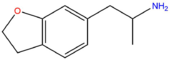
|
| 6-MAPB | H | H | H | H | CH2CH(NHCH3)CH3 | H | 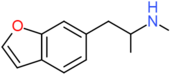
|
| 6-MAPDB | H2 | H2 | H | H | CH2CH(NHCH3)CH3 | H | 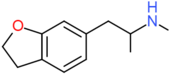
|
| bk-6-MAPB | H | H | H | H | COCH(NHCH3)CH3 | H | 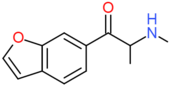
|
| 6-EAPB | H | H | H | H | CH2CH(NHCH2CH3)CH3 | H | 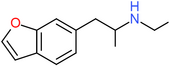
|
| 6-EAPDB | H2 | H2 | H | H | CH2CH(NHCH2CH3)CH3 | H | 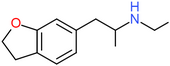
|
| 6-MBPB | H | H | H | H | CH2CH(NHCH3)CH2CH3 | H | 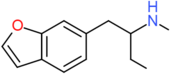
|
| F-2 | CH3 | H2 | H | OCH3 | CH2CH(NH2)CH3 | H | 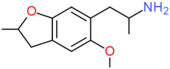
|
| F-22 | (CH3)2 | H2 | H | OCH3 | CH2CH(NH2)CH3 | H | 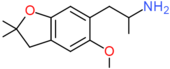
|
| 7-APB | H | H | H | H | H | CH2CH(NH2)CH3 | 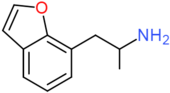
|
| 4-I-5-MeO-7-APDB | H2 | H2 | I | OCH3 | H | CH2CH(NH2)CH3 | 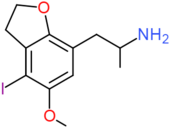
|