This is an unofficial archive of PsychonautWiki as of 2025-08-11T15:14:44Z. Content on this page may be outdated, incomplete, or inaccurate. Please refer to the original page for the most up-to-date information.
Substituted phenidates: Difference between revisions
Jump to navigation
Jump to search
>BronzeManul Created page, addition of pharmacology would be appreciated |
>Dextromethorphan |
||
| Line 17: | Line 17: | ||
! scope="col" | '''Structure''' | ! scope="col" | '''Structure''' | ||
|- | |- | ||
| [[Methylphenidate]] || H || H || H || H || H || | | [[Methylphenidate]] || H || H || H || H || H || CH<sub>3</sub> || [[File:Methyllphenidate.png|170px]] | ||
|- | |- | ||
| [[Ethylphenidate]] || H || H || H || H || H || | | [[Ethylphenidate]] || H || H || H || H || H || CH<sub>2</sub>CH<sub>3</sub> || [[File:Ethyllphenidate.png|170px]] | ||
|- | |- | ||
| [[Isopropylphenidate]] || H || H || H || H || H || | | [[Isopropylphenidate]] || H || H || H || H || H || CH(CH<sub>3</sub>)<sub>2</sub> || [[File:Isopropylphenidate.png|170px]] | ||
|- | |- | ||
| [[Propylphenidate]] || H || H || H || H || H || | | [[Propylphenidate]] || H || H || H || H || H || CH<sub>2</sub>CH<sub>2</sub>CH<sub>3</sub> || [[File:Propylphenidate.png|170px]] | ||
|- | |- | ||
| [[3,4-CTMP]] || H || Cl || Cl || H || H || | | [[3,4-CTMP]] || H || Cl || Cl || H || H || CH<sub>3</sub> || [[File:34-ctmp.png|170px]] | ||
|- | |- | ||
| [[4F-MPH]] || H || H || F || H || H || | | [[4F-MPH]] || H || H || F || H || H || CH<sub>3</sub> || [[File:4-Fluoromethylphenidate.png|170px]] | ||
|- | |- | ||
| [[4F-EPH]] || H || H || F || H || H || | | [[4F-EPH]] || H || H || F || H || H || CH<sub>2</sub>CH<sub>3</sub> || [[File:4F-EPH.png|170px]] | ||
|- | |- | ||
| [[Methylnaphthidate]] || H || | | [[Methylnaphthidate]] || H || CH=CH- || CH=CH- || H || H || CH<sub>3</sub> || [[File:Methylnaphthidate.png|170px]] | ||
|- | |- | ||
| [[Ethylnaphthidate]] || H || | | [[Ethylnaphthidate]] || H || CH=CH- || CH=CH- || H || H || CH<sub>2</sub>CH<sub>3</sub> || [[File:Ethylnaphthidate.png|170px]] | ||
|- | |- | ||
|} | |} | ||
Revision as of 06:17, 25 October 2016
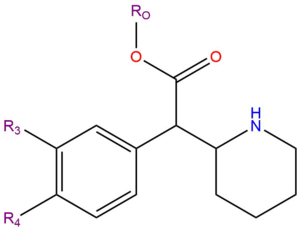
Substituted phenidates, also known as phenidates, are a class of chemicals that include compounds with psychoactive effects.
Chemistry
Substituted phenidates are a chemical class based upon the molecule Methylphenidate. Methylphenidate is made up of a phenethylamine molecule with a carbon chain substitution at the Rα position that links to the RN position forming a piperidine ring, and a substitution at the Rβ position of methyl acetate.
List of substituted phenidates
| Compound | R2 | R3 | R4 | R5 | R6 | RO | Structure |
|---|---|---|---|---|---|---|---|
| Methylphenidate | H | H | H | H | H | CH3 | 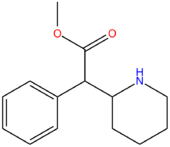
|
| Ethylphenidate | H | H | H | H | H | CH2CH3 | 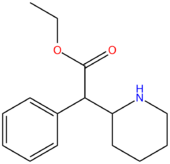
|
| Isopropylphenidate | H | H | H | H | H | CH(CH3)2 | 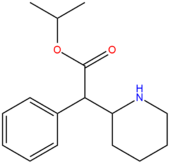
|
| Propylphenidate | H | H | H | H | H | CH2CH2CH3 | 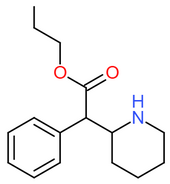
|
| 3,4-CTMP | H | Cl | Cl | H | H | CH3 | 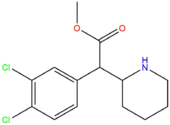
|
| 4F-MPH | H | H | F | H | H | CH3 | 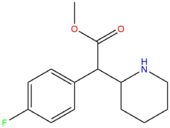
|
| 4F-EPH | H | H | F | H | H | CH2CH3 | 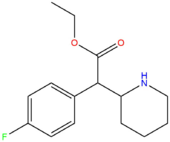
|
| Methylnaphthidate | H | CH=CH- | CH=CH- | H | H | CH3 | 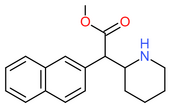
|
| Ethylnaphthidate | H | CH=CH- | CH=CH- | H | H | CH2CH3 | 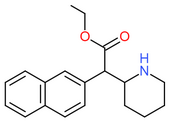
|