ΑMT: Difference between revisions
>Oskykins No edit summary |
>Oskykins m Text replacement - " research chemical " to " research chemical " |
||
| Line 88: | Line 88: | ||
Erowid has received "a handful of unverifiable reports of hospitalization after high-dose (over 60 mg oral) AMT ingestion."<ref name="amt">AMT (Alphamethyltryptamine, IT-290) Fatalities / Deaths by Erowid | https://www.erowid.org/chemicals/amt/amt_death.shtml</ref> There is one reported death from AMT which was reported in February 2003 by the Miami-Dade County Medical Examiner Department, but it is not known how much was taken.<ref name="amt2">Boland DM, Andollo W, Hime GW, Hearn WL. “Fatality due to acute alpha-methyltryptamine intoxication”. J Anal Toxicol. 2005 Jul-Aug;29(5):394-7. | https://www.erowid.org/references/refs_view.php?ID=6603</ref> | Erowid has received "a handful of unverifiable reports of hospitalization after high-dose (over 60 mg oral) AMT ingestion."<ref name="amt">AMT (Alphamethyltryptamine, IT-290) Fatalities / Deaths by Erowid | https://www.erowid.org/chemicals/amt/amt_death.shtml</ref> There is one reported death from AMT which was reported in February 2003 by the Miami-Dade County Medical Examiner Department, but it is not known how much was taken.<ref name="amt2">Boland DM, Andollo W, Hime GW, Hearn WL. “Fatality due to acute alpha-methyltryptamine intoxication”. J Anal Toxicol. 2005 Jul-Aug;29(5):394-7. | https://www.erowid.org/references/refs_view.php?ID=6603</ref> | ||
The toxicity and long-term health effects of recreational AMT use do not seem to have been studied in any scientific context and the exact [[Toxicity::toxic dose is unknown]]. This is because AMT is a research chemical with very little history of human usage. Anecdotal evidence from people who have tried AMT within the [[psychedelic]] community suggests that there are no negative health effects attributed to simply trying the drug by itself at low to moderate doses or using it very sparingly (but nothing can be completely guaranteed). [https://www.google.com/ Independent research] should always be done to ensure that a combination of two or more substances is safe before consumption. | The toxicity and long-term health effects of recreational AMT use do not seem to have been studied in any scientific context and the exact [[Toxicity::toxic dose is unknown]]. This is because AMT is a [[research chemical]] with very little history of human usage. Anecdotal evidence from people who have tried AMT within the [[psychedelic]] community suggests that there are no negative health effects attributed to simply trying the drug by itself at low to moderate doses or using it very sparingly (but nothing can be completely guaranteed). [https://www.google.com/ Independent research] should always be done to ensure that a combination of two or more substances is safe before consumption. | ||
It is worth noting that AMT's analogue [[αET]] has been shown to produce long-lasting serotonergic neurotoxicity at very high doses.<ref>Reduction in brain serotonin markers by α-ethyltryptamine (Monase) | http://www.sciencedirect.com/science/article/pii/001429999190686K</ref> It is possible that AMT could cause the same neurotoxicity at high dosages or with repeated long-term use. | It is worth noting that AMT's analogue [[αET]] has been shown to produce long-lasting serotonergic neurotoxicity at very high doses.<ref>Reduction in brain serotonin markers by α-ethyltryptamine (Monase) | http://www.sciencedirect.com/science/article/pii/001429999190686K</ref> It is possible that AMT could cause the same neurotoxicity at high dosages or with repeated long-term use. | ||
Revision as of 11:08, 21 February 2016
| ΑMT | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 |
|||||||||||||||||||||||||||||
| Chemical Nomenclature | |||||||||||||||||||||||||||||
| Common names | AMT, αMT, Indopan | ||||||||||||||||||||||||||||
| Substitutive name | α-Methyltryptamine, alpha-methyltryptamine | ||||||||||||||||||||||||||||
| Systematic name | 1-(1H-indol-3-yl)propan-2-amine | ||||||||||||||||||||||||||||
| Class Membership | |||||||||||||||||||||||||||||
| Psychoactive class | Entactogen / Psychedelic | ||||||||||||||||||||||||||||
| Chemical class | Tryptamine | ||||||||||||||||||||||||||||
| Routes of Administration | |||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||
| Interactions | |||||||||||||||||||||||||||||
| Alcohol | |||||||||||||||||||||||||||||
| Caffeine | |||||||||||||||||||||||||||||
| Cannabis | |||||||||||||||||||||||||||||
| 2C-T-x | |||||||||||||||||||||||||||||
| 2C-x | |||||||||||||||||||||||||||||
| 5-MeO-xxT | |||||||||||||||||||||||||||||
| Amphetamines | |||||||||||||||||||||||||||||
| Cocaine | |||||||||||||||||||||||||||||
| DOx | |||||||||||||||||||||||||||||
| DXM | |||||||||||||||||||||||||||||
| MAOIs | |||||||||||||||||||||||||||||
| MDMA | |||||||||||||||||||||||||||||
| Mescaline | |||||||||||||||||||||||||||||
| MXE | |||||||||||||||||||||||||||||
| 25x-NBOMe | |||||||||||||||||||||||||||||
| PCP | |||||||||||||||||||||||||||||
| SSRIs | |||||||||||||||||||||||||||||
| Tramadol | |||||||||||||||||||||||||||||
| Summary sheet: AMT |
α-Methyltryptamine (αMT, AMT, Indopan) is a psychedelic, stimulant, and entactogen drug of the tryptamine class.[1] It was originally developed as an antidepressant by workers at a Michigan pharmaceutical manufacturing company known as Upjohn in the 1960s.[2] In the 1960's, AMT was prescribed in 5 - 10 milligram doses as an antidepressant in the Soviet Union under the trade name Indopan.[3]
Erowid has received "a handful of unverifiable reports of hospitalization after high-dose (over 60 mg oral) AMT ingestion."[4] There is one reported death from AMT which was reported in February 2003 by the Miami-Dade County Medical Examiner Department, but it is not known how much was taken.[5]
Chemistry
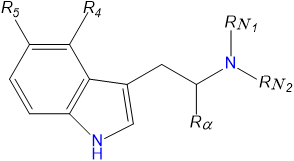
AMT or α-Methyltryptamine is a synthetic indole alkaloid molecule of the tryptamine class. Tryptamines share a core structure comprised of a bicylic indole heterocycle attached at R3 to an amino group via an ethyl side chain. AMT is substituted at the alpha carbon Rα of its tryptamine backbone with a methyl group. AMT is found in freebase form as a racemate of its (R-) and (S-) enantiomers. [6]
Pharmacology
AMT's psychedelic effects are believed to come from its efficacy at the 5-HT2A receptor as a partial agonist.
AMT also acts as a releasing agent of serotonin, noradrenaline, and dopamine.[7][8] It also acts as a very weak, non-selective RIMA in-vitro[9] and in-vivo.[10], but this is unlikely to be very significant (if at all) with typical doses.
However, the role of these interactions and how they result in the psychedelic experience continues to remain elusive.
Subjective effects
The effects listed below are based upon the subjective effects index and personal experiences of PsychonautWiki contributors. The listed effects will rarely (if ever) occur all at once, but heavier dosages will increase the chances and are more likely to induce a full range of effects.
Physical effects
The physical effects of this drug may be overly intense for those who are not already experienced with psychedelics.
- Spontaneous tactile sensations - AMT's "body high" can be described as an intense and constant all-encompassing sensation. In comparison to other psychedelics, this sensation does not manifest itself in the form of a continuously shifting tingling sensation that travels up and down the body spontaneously; it is instead felt as an extended, unchanging activation of every nerve ending on the body that lasts throughout the entire duration of the trip. This continuous sensation is immensely pleasurable but can become overwhelmingly intense and almost a burden at higher levels.
- Stimulation - In terms of its effects on the physical energy levels of the tripper, AMT is very stimulating, resulting in jaw clenching and a shakiness and unsteadiness of the hands. The stimulation encourages trippers to move around, run, dance, climb or generally engage in physical activities. In comparison, other more commonly used psychedelics such as psilocybin are generally sedating and relaxed.
- Nausea - In terms of the physical discomfort experienced on this substance, moderate to extreme nausea is almost consistently reported when consumed at any dosage. This either passes once the tripper has vomited or gradually fades by itself as the peak sets in.
- Difficulty urinating - A slight difficulty urinating is occasionally present.
- Headaches - Many people report headaches towards the end of the experience
- Temperature regulation suppression
- Increased heart rate
- Increased perspiration
- Increased blood pressure
- Pupil dilation
Cognitive effects
In comparison to more traditional psychedelics such as LSD, DMT and Psilocin, the AMT head space is described as not nearly as deep, insightful or profound.
The total sum of these cognitive components regardless of the setting generally includes:
- Analysis enhancement - This component is introspection dominant consistently manifested only in the context of a non-social setting in which the user is alone.
- Empathy, love and sociability enhancement - This component is consistently manifested only in the context of social settings in which one is within the company of others. These feelings of sociability, love and empathy are a little weaker and less sharp than those found on substances such as MDMA and 2C-B but still prove strong enough to provide long-lasting therapeutic effects.
- Thought acceleration
- Thought connectivity
- Immersion enhancement
- Empathy, love and sociability enhancement
- Current mind state enhancement
- Euphoria
- Conceptual thinking
- Memory suppression
- Ego death
- Time distortion
- Wakefulness
Visual effects
The visual effects of AMT are mostly present only when large doses have been consumed and are proportionally mild in comparison to the intensity of its accompanying cognitive and physical effects when compared to substances such as LSD and psilocin.
Enhancements
Distortions
- Drifting (Melting, Flowing, Breathing and morphing) - In comparison to other psychedelics, this effect can be described as highly detailed, slow and smooth in motion, static in appearance and unrealistic/cartoon-like in style.
- Symmetrical texture repetition - In comparison to more commonly used psychedelics such as LSD and psilocin, this effect is significantly less intricate and complex although it is still very distinct in its presence.
- Tracers
- After images
- Colour shifting
- Scenery slicing
The visual geometry that is present throughout this trip can be described as more similar in appearance to that of Psilocin and 2C-E than LSD. At lower levels it can appear to be bland and simplistic in complexity but becomes equal in terms of intricacy and depth to that of any of the classical psychedelics at higher dosages. It can be comprehensively described with its variations as intricate in complexity (at heavy dosages), abstract in form, organic in feel, structured in organization, brightly lit, multicoloured in scheme, glossy in shading, equal in soft and sharp edges, small in size, fast in speed, smooth in motion, equal in round and angular corners, non-immersive in depth, and consistent in intensity. At higher dosages, the visual geometry is significantly more likely to result in states of Level 8B geometry over Level 8A.
Hallucinatory states
- Transformations
- Internal hallucinations (autonomous entities; settings, sceneries, and landscapes; alterations in perspective and scenarios and plots) - This particular effect is uncommon during the first half of the trip but capable of manifesting itself towards the end of the experience, particularly if sleep deprivation starts to take its toll due to the abnormally long duration. The internal hallucinations are more common within dark environments and can be comprehensively described through their variations as lucid in believability, fixed in style, new experiences in content, autonomous in controllability, geometry-based in style and almost exclusively of a personal, religious, spiritual, science-fiction, fantasy, surreal, nonsensical or transcendental nature in their overall theme.
Auditory effects
Toxicity and harm potential
Erowid has received "a handful of unverifiable reports of hospitalization after high-dose (over 60 mg oral) AMT ingestion."[4] There is one reported death from AMT which was reported in February 2003 by the Miami-Dade County Medical Examiner Department, but it is not known how much was taken.[5]
The toxicity and long-term health effects of recreational AMT use do not seem to have been studied in any scientific context and the exact toxic dose is unknown. This is because AMT is a research chemical with very little history of human usage. Anecdotal evidence from people who have tried AMT within the psychedelic community suggests that there are no negative health effects attributed to simply trying the drug by itself at low to moderate doses or using it very sparingly (but nothing can be completely guaranteed). Independent research should always be done to ensure that a combination of two or more substances is safe before consumption.
It is worth noting that AMT's analogue αET has been shown to produce long-lasting serotonergic neurotoxicity at very high doses.[11] It is possible that AMT could cause the same neurotoxicity at high dosages or with repeated long-term use.
Tolerance and addiction potential
AMT is not habit-forming and the desire to use it can actually decrease with use. It is most often self-regulating.
Tolerance to the effects of AMT are built almost immediately after ingestion. After that it takes about 3 days for the tolerance to be reduced to half and 7 days to be back at baseline (in the absence of further consumption). AMT presents cross-tolerance with [[Cross-tolerance::all psychedelics]], meaning that after the consumption of AMT all psychedelics will have a reduced effect.
Interactions
Deaths from AMT are rare but, as a powerful monoamine reuptake inhibitor, injury could occur when excessive doses are taken or when it is taken with drugs such as MAOIs, RIMAs, stimulants and any substance which act as a releasing agent or reuptake inhibitor of neurotransmitters such as serotonin and dopamine.[12]
Legal issues
- Australia: Sale and possession of AMT is illegal.
- Germany: Sale and possession of AMT is illegal.
- Greece: Sale and possession of AMT is illegal.
- Japan: Sale and possession of AMT is illegal.
- Russia: Sale and possession of AMT is illegal.
- Sweden: Sale and possession of AMT is illegal.[13]
- USA: AMT is a Schedule I drug.[14]
- UK: AMT is a Class A drug and the sale and possession are illegal. See "the Misuse of Drugs Act 1971 (Amendment) (No. 2) Order 2014"[15]
- Canada: Canada has no mention of this substance in the Controlled Drugs and Substances Act.[16]
- Latvia: AMT is a Schedule I drug.[17]
Experience reports
Anecdotal reports which describe the effects of this compound within our experience index include:
- Experience:12 mg AMT - Nicely Surprised
- Experience:30mg - Horrible bodyload
- Experience:30mg - Psychostimulant egodeath
- Experience:aMT (70 mg, oral) - Testing AMT to the limit
See also
External links
References
- ↑ Erowid Online Books : TIHKAL - #48 a-MT | http://www.erowid.org/library/books_online/tihkal/tihkal48.shtml
- ↑ US Patent 3296072 - Method of Treating Mental Depression
- ↑ AMT's TiHKAL entry by Alexander Shulgan (IsomerDesigns) https://isomerdesign.com/PiHKAL/read.php?domain=tk&id=48
- ↑ 4.0 4.1 AMT (Alphamethyltryptamine, IT-290) Fatalities / Deaths by Erowid | https://www.erowid.org/chemicals/amt/amt_death.shtml
- ↑ 5.0 5.1 Boland DM, Andollo W, Hime GW, Hearn WL. “Fatality due to acute alpha-methyltryptamine intoxication”. J Anal Toxicol. 2005 Jul-Aug;29(5):394-7. | https://www.erowid.org/references/refs_view.php?ID=6603
- ↑ http://isomerdesign.com/PiHKAL/read.php?id=48
- ↑ The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain | http://linkinghub.elsevier.com/retrieve/pii/S0014-2999(06)01381-1
- ↑ In vitro screening of psychoactive drugs by [(35)S]GTPgammaS binding in rat brain membranes | https://www.jstage.jst.go.jp/article/bpb/30/12/30_12_2328/_article
- ↑ Studies of Monoamine Oxidase and Semicarbazide-Sensitive Amine Oxidase II. Inhibition by α-Methylated Substrate-Analogue Monoamines, α-Methyltryptamine, α-Methylbenzylamine and Two Enantiomers of α-Methylbenzylamine | https://www.jstage.jst.go.jp/article/jphs1951/41/2/41_2_191/_article
- ↑ THE EFFECT OF THREE TRYPTAMINE DERIVATIVES ON SEROTONIN METABOLISM IN VITRO AND IN VIVO | http://jpet.aspetjournals.org/content/127/2/110.short
- ↑ Reduction in brain serotonin markers by α-ethyltryptamine (Monase) | http://www.sciencedirect.com/science/article/pii/001429999190686K
- ↑ Monoamine oxidase inhibitors, opioid analgesics and serotonin toxicity | http://bja.oxfordjournals.org/content/95/4/434
- ↑ Svensk författningssamling Förordning om ändring i förordningen (1999:58) om förbud mot vissa hälsofarliga varor | http://www.notisum.se/rnp/sls/sfs/20050026.pdf
- ↑ Drug Enforcement Administration | http://webcache.googleusercontent.com/search?q=cache:http://www.deadiversion.usdoj.gov/drug_chem_info/amt.pdf
- ↑ the Misuse of Drugs Act 1971 (Amendment) (No. 2) Order 2014 | http://www.legislation.gov.uk/uksi/2014/3271/article/4/made
- ↑ CSDA | http://isomerdesign.com/Cdsa/schedule.php?structure=C
- ↑ Noteikumi par Latvijā kontrolējamajām narkotiskajām vielām, psihotropajām vielām un prekursoriem (Triptamīni) | http://likumi.lv/doc.php?id=121086