Gabapentinoids: Difference between revisions
>Azed m Added a citation |
>Azed m Added two cites. Changed wording. |
||
| Line 2: | Line 2: | ||
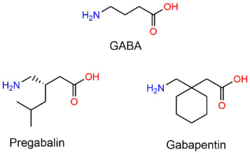
[[File:Gabapentinoidsstructure.png|250px|thumbnail|Diagram showing the structural similarities of [[GABA|gamma-aminobutyric acid]] (GABA), [[pregabalin]] and [[gabapentin]].]] | [[File:Gabapentinoidsstructure.png|250px|thumbnail|Diagram showing the structural similarities of [[GABA|gamma-aminobutyric acid]] (GABA), [[pregabalin]] and [[gabapentin]].]] | ||
'''Gabapentinoids''' are a chemical class of [[psychoactive substances]] derived from [[GABA|gamma-aminobutyric acid]] (GABA). | '''Gabapentinoids''' are a chemical class of [[psychoactive substances]] derived from [[GABA|gamma-aminobutyric acid]] (GABA).{{citation needed}} Members of this class include [[gabapentin]], [[F-phenibut]], [[phenibut]] and [[pregabalin]]. | ||
Gabapentinoids are commonly prescribed for epilepsy, neuropathic pain, and [[restless legs syndrome]]. [[Subjective effects]] include [[sedation]], [[muscle relaxation]], and [[anxiety suppression]]. | Gabapentinoids are commonly prescribed for epilepsy, neuropathic pain, and [[restless legs syndrome]]. [[Subjective effects]] include [[sedation]], [[muscle relaxation]], and [[anxiety suppression]]. | ||
| Line 12: | Line 12: | ||
==Pharmacology== | ==Pharmacology== | ||
Gabapentinoids act by | Gabapentinoids act by inhibiting the α2δ subunit-containing voltage-dependent calcium [[receptor#Ion_channels|channels]] (VGCCs).<ref>Patel, R., & Dickenson, A. H. (2016). Mechanisms of the gabapentinoids and α2δ‐1 calcium channel subunit in neuropathic pain. Pharmacology research & perspectives, 4(2).https://doi.org/10.1002/prp2.205</ref> While all gabapentinoids block the α2δ channels, they also have unique pharmacological characteristics such as enzyme inhibition.<ref>Goldlust, A., Su, T. Z., Welty, D. F., Taylor, C. P., & Oxender, D. L. (1995). Effects of anticonvulsant drug gabapentin on the enzymes in metabolic pathways of glutamate and GABA. Epilepsy research, 22(1), 1-11.https://doi.org/10.1016/0920-1211(95)00028-9</ref> | ||
==Examples== | ==Examples== | ||
*[[F-Phenibut]] | *[[F-Phenibut]] | ||
*[[Gabapentin]] | *[[Gabapentin]] | ||
| Line 22: | Line 21: | ||
==See also== | ==See also== | ||
*[[Responsible use]] | *[[Responsible use]] | ||
*[[Depressants]] | *[[Depressants]] | ||
| Line 30: | Line 28: | ||
==External links== | ==External links== | ||
*[https://en.wikipedia.org/wiki/Gabapentinoid Gabapentinoid (Wikipedia)] | *[https://en.wikipedia.org/wiki/Gabapentinoid Gabapentinoid (Wikipedia)] | ||
| Line 38: | Line 35: | ||
[[Category:Chemical class]] | [[Category:Chemical class]] | ||
[[Category:Pharmacology]] | [[Category:Pharmacology]] | ||
Revision as of 04:35, 20 November 2020
 |
This article is a stub. As such, it may contain incomplete or wrong information. You can help by expanding it. |
Gabapentinoids are a chemical class of psychoactive substances derived from gamma-aminobutyric acid (GABA).[citation needed] Members of this class include gabapentin, F-phenibut, phenibut and pregabalin.
Gabapentinoids are commonly prescribed for epilepsy, neuropathic pain, and restless legs syndrome. Subjective effects include sedation, muscle relaxation, and anxiety suppression.
Gabapentinoids can be dangerous when mixed with other depressants such as benzodiazepines, alcohol and opioids.
Chemistry
Gabapentinoids are close structural relatives, and are all 3-substituted derivatives of GABA, the differences being the addition of a cyclohexyl group on the GABA chain in the case of gabapentin, the substitution of that cyclohexyl group for an isobutyl group in the case of pregabalin, and the substitution of that isobutyl group with a cyclic phenyl ring in the case of phenibut. Hence, they are GABA analogues, as well as γ-amino acids.[1][2]
Pharmacology
Gabapentinoids act by inhibiting the α2δ subunit-containing voltage-dependent calcium channels (VGCCs).[3] While all gabapentinoids block the α2δ channels, they also have unique pharmacological characteristics such as enzyme inhibition.[4]
Examples
See also
External links
References
- ↑ Elaine Wyllie; Gregory D. Cascino; Barry E. Gidal; Howard P. Goodkin (17 February 2012). Wyllie's Treatment of Epilepsy: Principles and Practice. Lippincott Williams & Wilkins. p. 423. ISBN 978-1-4511-5348-4.
- ↑ Honorio Benzon; James P. Rathmell; Christopher L. Wu; Dennis C. Turk; Charles E. Argoff; Robert W Hurley (11 September 2013). Practical Management of Pain. Elsevier Health Sciences. p. 1006. ISBN 978-0-323-17080-2.
- ↑ Patel, R., & Dickenson, A. H. (2016). Mechanisms of the gabapentinoids and α2δ‐1 calcium channel subunit in neuropathic pain. Pharmacology research & perspectives, 4(2).https://doi.org/10.1002/prp2.205
- ↑ Goldlust, A., Su, T. Z., Welty, D. F., Taylor, C. P., & Oxender, D. L. (1995). Effects of anticonvulsant drug gabapentin on the enzymes in metabolic pathways of glutamate and GABA. Epilepsy research, 22(1), 1-11.https://doi.org/10.1016/0920-1211(95)00028-9