Diarylethylamines: Difference between revisions
>Unity m Add paper |
>Unity →Literature: Added research paper |
||
| Line 69: | Line 69: | ||
* Wallach, J., Kang, H., Colestock, T., Morris, H., Bortolotto, Z. A., Collingridge, G. L., ... & Adejare, A. (2016). Pharmacological investigations of the dissociative ‘legal highs’ diphenidine, methoxphenidine and analogues. PLoS One, 11(6), e0157021. https://doi.org/10.1371/journal.pone.0157021 | * Wallach, J., Kang, H., Colestock, T., Morris, H., Bortolotto, Z. A., Collingridge, G. L., ... & Adejare, A. (2016). Pharmacological investigations of the dissociative ‘legal highs’ diphenidine, methoxphenidine and analogues. PLoS One, 11(6), e0157021. https://doi.org/10.1371/journal.pone.0157021 | ||
* Morris, H., & Wallach, J. (2014). From PCP to MXE: A comprehensive review of the non-medical use of dissociative drugs. Drug Testing and Analysis, 6(7–8), 614–632. https://doi.org/10.1002/dta.1620 | * Morris, H., & Wallach, J. (2014). From PCP to MXE: A comprehensive review of the non-medical use of dissociative drugs. Drug Testing and Analysis, 6(7–8), 614–632. https://doi.org/10.1002/dta.1620 | ||
* Wallach, J., & Brandt, S. D. (2018). 1, 2-Diarylethylamine-and Ketamine-Based New Psychoactive Substances. In New Psychoactive Substances (pp. 305-352). Springer, Cham. http://dx.doi.org/10.1007/164_2018_148 | |||
==References== | ==References== | ||
<references /> | <references /> | ||
Revision as of 03:50, 20 April 2019
 |
This article is a stub. As such, it may contain incomplete or wrong information. You can help by expanding it. |
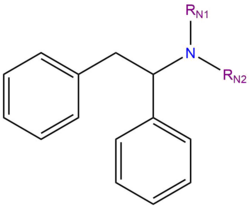
Diarylethylamines are a class of psychoactive substances that produce dissociative effects when administered. Their characteristic effects are similar to those of arylcyclohexylamine dissociatives like PCP or ketamine, although they differ in their chemical structure.
Diarylethylamines are examples of contemporary designer drugs specifically chosen to mimic and/or replace the functional and structural features of commonly used illicit substances. They have been marketed on the online research chemicals market as a replacement for MXE and other dissociatives.
Very little is known about the human pharmacology, metabolism, and toxicity of these compounds. Many reports suggest that they may pose different and more pronounced risks than traditional dissociatives.
Pharmacology
| Compound | IC50 ± SEM (nM) | Ki ± SEM (nM) |
|---|---|---|
| PCP[2] | 91 ± 1.3 | 57.9 ± 0.8 |
| Ketamine | 508.5 ± 30.1 | 323.9 ± 19.2 |
| Memantine | 594.2 ± 41.3 | 378.4 ± 26.3 |
| (+)-MK-801 | 4.1 ± 1.6 | 2.5 ± 1.0 |
| DPH (Diphenidine) | 28.6 ± 3.5 | 18.2 ± 2.2 |
| 2-MXP (Methoxphenidine) | 56.5 ± 5.8 | 36.0 ± 3.7 |
| 3-MXP | 30.3 ± 2.6 | 19.3 ± 1.7 |
| 4-MXP | 723.8 ± 69.9 | 461.0 ± 44.5 |
| 2-Cl-DPH | 14.6 ± 2.1 | 9.3 ± 1.3 |
| NMDAR binding affinites determined using [3H]-(+)-MK-801 in rat forebrain.[3] | ||
Examples
See also
Literature
- Wallach, J., Kang, H., Colestock, T., Morris, H., Bortolotto, Z. A., Collingridge, G. L., ... & Adejare, A. (2016). Pharmacological investigations of the dissociative ‘legal highs’ diphenidine, methoxphenidine and analogues. PLoS One, 11(6), e0157021. https://doi.org/10.1371/journal.pone.0157021
- Morris, H., & Wallach, J. (2014). From PCP to MXE: A comprehensive review of the non-medical use of dissociative drugs. Drug Testing and Analysis, 6(7–8), 614–632. https://doi.org/10.1002/dta.1620
- Wallach, J., & Brandt, S. D. (2018). 1, 2-Diarylethylamine-and Ketamine-Based New Psychoactive Substances. In New Psychoactive Substances (pp. 305-352). Springer, Cham. http://dx.doi.org/10.1007/164_2018_148