GHB: Difference between revisions
>Josikins |
>Josikins |
||
| Line 74: | Line 74: | ||
==Toxicity and harm potential== | ==Toxicity and harm potential== | ||
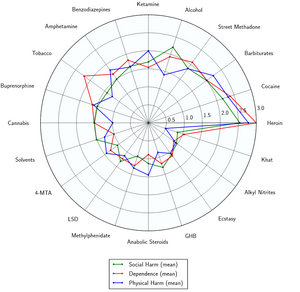
[[File:harmchart.png|thumb|right|300px|Radar plot showing relative physical harm, social harm, and dependence of GHB<ref>Development of a rational scale to assess the harm of drugs of potential misuse | http://www.sciencedirect.com/science/article/pii/S0140673607604644</ref>]] | |||
GHB is considered as a very safe, benign and non toxic substance when used responsibly. As an endogenous regulator of energy metabolism and a natural neurotransmitter, it is well-known to the brain and organs. They are used to its effects and have highly efficient systems for metabolizing it safely.<ref> Psychotherapeutic Drugs. 1340-1375. Bibliographic information missing.</ref> The substance is eliminated (that is, back to baseline levels) in 2-4 hours and continues to be so even after twice-daily doses for a week.<ref>Ferrara, SD. Zotti, S. Tedeschi, L. Frison, G. Palatini, P. et al.. "Pharmacokinetics of gamma-hydroxybutyric acid in alcohol dependent. . .". British Journal of Clinical Pharmacology. 1992. 34. 231-235. R 31 B 93. . </ref> In one European study, no adverse effects were reported after several years of hypnotic* use.<ref>Laborit H . "Correlations between protein and serotonin synthesis during various activities of the central nervous system (slow and desynchronized sleep, learning and memory, sexual activity, morphine tolerance, aggressiveness, and pharmacological action of sodium ga. RESEARCH COMMUNICATIONS IN CHEMICAL PATHOLOGY AND PHARMACOLOGY. 1972. 3(1). </ref> The LD50 is far above the active dosage, and there is literally no danger of acute toxicity. | GHB is considered as a very safe, benign and non toxic substance when used responsibly. As an endogenous regulator of energy metabolism and a natural neurotransmitter, it is well-known to the brain and organs. They are used to its effects and have highly efficient systems for metabolizing it safely.<ref> Psychotherapeutic Drugs. 1340-1375. Bibliographic information missing.</ref> The substance is eliminated (that is, back to baseline levels) in 2-4 hours and continues to be so even after twice-daily doses for a week.<ref>Ferrara, SD. Zotti, S. Tedeschi, L. Frison, G. Palatini, P. et al.. "Pharmacokinetics of gamma-hydroxybutyric acid in alcohol dependent. . .". British Journal of Clinical Pharmacology. 1992. 34. 231-235. R 31 B 93. . </ref> In one European study, no adverse effects were reported after several years of hypnotic* use.<ref>Laborit H . "Correlations between protein and serotonin synthesis during various activities of the central nervous system (slow and desynchronized sleep, learning and memory, sexual activity, morphine tolerance, aggressiveness, and pharmacological action of sodium ga. RESEARCH COMMUNICATIONS IN CHEMICAL PATHOLOGY AND PHARMACOLOGY. 1972. 3(1). </ref> The LD50 is far above the active dosage, and there is literally no danger of acute toxicity. | ||
Revision as of 23:00, 1 May 2014
GHB, also known as γ-Hydroxybutyric acid and 4-hydroxybutanoic acid, is a naturally occurring substance with depressant effects which is found in the human central nervous system, as well as in wine, beef, small citrus fruits, and in small amounts in almost all animals.[1]
GHB as the sodium salt, known as sodium oxybate (INN) or by the trade name Xyrem,[2] is used to treat cataplexy[3] and excessive daytime sleepiness in patients with narcolepsy. It has also been used in a medical setting as a general anesthetic, to treat conditions such as insomnia, clinical depression, narcolepsy, and alcoholism, and to improve athletic performance. It is also used as an intoxicant (illegally in many jurisdictions) or as a date rape drug.[4]
GHB is naturally produced in the human body's cells. As a supplement or drug, it is used most commonly in the form of a salt. GHB is also produced as a result of fermentation, and so is found in small quantities in some beers and wines. Succinic semialdehyde dehydrogenase deficiency is a disease that causes GHB to accumulate in the blood.
Chemistry
Gamma-Hydroxybutyric acid is comprised of butyric acid, a 4-carbon fatty acid chain with a -carboxyl group at one end, and a hydroxy- functional at the gamma position. GHB is most commonly found in the form of its sodium salt.
Pharmacology
GHB has at least two distinct binding sites[5] in the central nervous system. GHB is an agonist at the newly characterized GHB receptor, which is excitatory,[6][7] and it is a weak agonist at the GABAB receptor, which is inhibitory.[8]
GHB induces the accumulation of either a derivative of tryptophan or tryptophan itself, possibly by increasing tryptophan transport across the blood–brain barrier. GHB-induced stimulation may be due to this increase in tryptophan transport to the brain and in its uptake by serotonergic cells. As the serotonergic system may be involved in the regulation of sleep, mood, and anxiety, the stimulation of this system by high doses of GHB may be involved in certain neuropharmacological events induced by GHB administration.
However, at therapeutic doses, GHB reaches much higher concentrations in the brain and activates GABAB receptors, which are primarily responsible for its sedative effects.[9] GHB's sedative effects are blocked by GABAB antagonists.
There has been somewhat limited research into the GHB receptor; however, there is evidence that activation of the GHB receptor in some brain areas results in the release of glutamate, the principal excitatory neurotransmitter.[10] Drugs that selectively activate the GHB receptor cause absence seizures in high doses, as do GHB and GABAB agonists.[11]
Activation of both the GHB receptor and GABAB is responsible for the addictive profile of GHB. GHB's effect on dopamine release is biphasic.[12] This means that while low concentrations stimulate dopamine release via the GHB receptor.[13] Higher concentrations inhibit dopamine release via GABAB receptors.[14] After an initial phase of inhibition, dopamine release is then increased via the GHB receptor.
This explains the paradoxical mix of sedative and stimulatory properties of GHB, as well as the so-called "rebound" effect, experienced by individuals using GHB as a sleeping agent, wherein they awake suddenly after several hours of GHB-induced deep sleep. That is to say that, over time, the concentration of GHB in the system decreases below the threshold for significant GABAB receptor activation and activates predominantly the GHB receptor, leading to wakefulness.
Subjective effects
Physical effects
The physical effects of GHB can be broken down into 7 components all of which progressively intensify proportional to dosage. These are described below and generally include:
- Stimulation and Sedation - At lower dosages GHB is physically stimulating, encouraging movement and wakefulness. At higher dosages however it becomes physically sedating, encouraging sleep and lethargy.
- Euphoria
- Loss of motor control
- Dizziness
- Dehydration
- Respiratory depression
Cognitive effects
The cognitive effects of GHB can be broken down into 6 components all of which progressively intensify proportional to dosage. It contains a large number of typical depressant cognitive effects which generally include:
- Increased empathy, love and sociability - Unlike alcohol which merely increases sociability through disinhibition, GHB presents strong entactogenic effects which although weaker than that of MDMA are still prominent and well defined.
- Disinhibition
- Suppression of information processing
- Thought deceleration
- Amnesia
- Euphoria
Toxicity and harm potential
GHB is considered as a very safe, benign and non toxic substance when used responsibly. As an endogenous regulator of energy metabolism and a natural neurotransmitter, it is well-known to the brain and organs. They are used to its effects and have highly efficient systems for metabolizing it safely.[16] The substance is eliminated (that is, back to baseline levels) in 2-4 hours and continues to be so even after twice-daily doses for a week.[17] In one European study, no adverse effects were reported after several years of hypnotic* use.[18] The LD50 is far above the active dosage, and there is literally no danger of acute toxicity.
Although GHB is thought to be perfectly safe to use on a semi regular basis at reasonable dosages. In multiple studies, excessive GHB use for extended periods of time has been found to impair spatial memory, working memory, learning and memory in rats with chronic administration.[19][20][21][22]
One study found that repeated administration of GHB to rats for 15 days drastically reduced the number of neurons and non-neuronal cells within the hippocampus and in the prefrontal cortex. With doses of 10 mg/kg of GHB, they were decreased by 61% in the hippocampus region and 32% in the prefrontal cortex, and with 100 mg/kg, they were decreased by 38% and 9%, respectively. This is interesting as it demonstrates contradicting effects on neuronal loss, with lower doses (10 mg/kg) producing the most neurotoxicity, and higher doses (100 mg/kg) producing less.
Tolerance and addiction potential
GHB is not thought to be habit forming although it is entirely possible for one to develop a psychological dependence. There does not appear to be a particularly powerful tolerance with GHB and because of this one rarely needs to increase the amount much beyond their start dosage unless using on an extremely regular basis.
Rats forced to consume massive doses of GHB will intermittently prefer GHB solution to water but, after experiments on rats, it was noted that "no rat showed any sign of withdrawal when GHB was finally removed at the end of the 20-week period" or during periods of voluntary abstinence.[23][24] Although there have been reported fatalities due to GHB withdrawal, reports are inconclusive and further research is needed.[25]
Interactions
Although safe by itself, GHB may be dangerous when combined with other depressants such as alcohol, benzodiazepines and opioids. A review of the details of 194 deaths attributed to or related to GHB over a ten-year period found that most were from respiratory depression caused by interaction with alcohol or other drugs.[26] In humans, GHB has been shown to inhibit the elimination rate of alcohol. This may explain the respiratory arrest that has been reported after ingestion of both drugs.[27]
Legal issues
- USA: GHB was placed on Schedule I of the Controlled Substances Act in March 2000. However, when sold as sodium oxybate, it is considered a Schedule III substance but with Schedule I trafficking penalties, one of several drugs that are listed in multiple schedules.[28]
- UK: GHB was made a class C drug in June 2003.
- Hong Kong: GHB is regulated under Schedule 1 of Hong Kong's Chapter 134 Dangerous Drugs Ordinance.
- New Zealand: GHB, 1,4-B and GBL are all Class B illegal drugs, along with any possible esters, ethers and aldehydes.
- Australia: GHB, 1,4-B and GBL are all Class B illegal drugs, along with any possible esters, ethers and aldehydes.
- Chile: GHB is a controlled drug under the law "Ley de substancias psicotropicas y estupefacientes" (psychotropic substances and narcotics).
- Norway: GHB is considered a narcotic and is only available by prescription under the trade name Xyrem.
- Switzerland: GHB is considered a narcotic and is only available by prescription under the trade name Xyrem.
See also
References
- ↑ Weil, Andrew; Winifred Rosen (1993). "Depressants". From Chocolate to Morphine (2nd ed.). Boston/New York: Houghton Mifflin Company. p. 77. ISBN 0-395-66079-3.
- ↑ http://www.reuters.com/finance/stocks/companyProfile?rpc=66&symbol=JAZZ.O
- ↑ Sodium Oxybate | http://www.nlm.nih.gov/medlineplus/druginfo/meds/a605032.html
- ↑ GHB, GBL and 1,4BD as Date Rape Drugs | http://web.archive.org/web/20120510151441/http://www.justice.gov/dea//ongoing/daterapep.html
- ↑ Gammahydroxybutyrate: An endogenous regulator of energy metabolism | http://www.sciencedirect.com/science/article/pii/S0149763489800533
- ↑ γ-Hydroxybutyric acid (GHB) and γ-aminobutyric acidB receptor (GABABR) binding sites are distinctive from one another: molecular evidence | http://www.sciencedirect.com/science/article/pii/S0028390804002527
- ↑ A mechanism for γ-hydroxybutyrate (GHB) as a drug and a substance of abuse | http://www.medecinesciences.org/articles/medsci/abs/2005/03/medsci2005213p284/medsci2005213p284.html
- ↑ A mechanism for γ-hydroxybutyrate (GHB) as a drug and a substance of abuse | http://www.medecinesciences.org/articles/medsci/abs/2005/03/medsci2005213p284/medsci2005213p284.html
- ↑ Drosophila GABAB receptors are involved in behavioral effects of γ-hydroxybutyric acid (GHB) | http://www.sciencedirect.com/science/article/pii/S0014299905007442
- ↑ Selective γ-hydroxybutyric acid receptor ligands increase extracellular glutamate in the hippocampus, but fail to activate G protein and to produce the sedative/hypnotic effect of γ-hydroxybutyric acid | http://onlinelibrary.wiley.com/doi/10.1046/j.1471-4159.2003.02037.x/abstract
- ↑ Selective γ-hydroxybutyric acid receptor ligands increase extracellular glutamate in the hippocampus, but fail to activate G protein and to produce the sedative/hypnotic effect of γ-hydroxybutyric acid | http://onlinelibrary.wiley.com/doi/10.1046/j.1471-4159.2003.02037.x/abstract
- ↑ Drosophila GABAB receptors are involved in behavioral effects of γ-hydroxybutyric acid (GHB) | http://www.sciencedirect.com/science/article/pii/S0014299905007442
- ↑ A specific gamma-hydroxybutyrate receptor ligand possesses both antagonistic and anticonvulsant properties | http://www.ncbi.nlm.nih.gov/pubmed/2173754
- ↑ Tonic GABA-ergic modulation of striatal dopamine release studied by in vivo microdialysis in the freely moving rat | http://www.sciencedirect.com/science/article/pii/001429999500369V
- ↑ Development of a rational scale to assess the harm of drugs of potential misuse | http://www.sciencedirect.com/science/article/pii/S0140673607604644
- ↑ Psychotherapeutic Drugs. 1340-1375. Bibliographic information missing.
- ↑ Ferrara, SD. Zotti, S. Tedeschi, L. Frison, G. Palatini, P. et al.. "Pharmacokinetics of gamma-hydroxybutyric acid in alcohol dependent. . .". British Journal of Clinical Pharmacology. 1992. 34. 231-235. R 31 B 93. .
- ↑ Laborit H . "Correlations between protein and serotonin synthesis during various activities of the central nervous system (slow and desynchronized sleep, learning and memory, sexual activity, morphine tolerance, aggressiveness, and pharmacological action of sodium ga. RESEARCH COMMUNICATIONS IN CHEMICAL PATHOLOGY AND PHARMACOLOGY. 1972. 3(1).
- ↑ Adolescent γ-hydroxybutyric acid exposure decreases cortical N-methyl-d-aspartate receptor and impairs spatial learning | http://www.sciencedirect.com/science/article/pii/S009130570400320X
- ↑ Effects of subchronic administration of gammahydroxybutyrate (GHB) on spatial working memory in rats | http://www.ncbi.nlm.nih.gov/pubmed/17296081
- ↑ γ-Hydroxybutyric Acid–Induced Cognitive Deficits in the Female Adolescent Rat | http://onlinelibrary.wiley.com/doi/10.1196/annals.1432.044/abstract
- ↑ Neurotoxic effects induced by gammahydroxybutyric acid (GHB) in male rats | http://journals.cambridge.org/action/displayAbstract?fromPage=online&aid=6137924
- ↑ Oral self-administration of γ-hydroxybutyric acid in the rat | http://www.sciencedirect.com/science/article/pii/0014299995004935
- ↑ http://www.lycaeum.org/~ghbfaq/dangerous.html
- ↑ Gamma-hydroxybutyrate: an emerging drug of abuse that causes physical dependence | http://onlinelibrary.wiley.com/doi/10.1111/j.1360-0443.1997.tb03640.x/abstract
- ↑ http://web.archive.org/web/20071203005230/http://www.aafs.org/pdf/Seattleabstracts06.pdf
- ↑ The role of gamma-hydroxybutyric acid in the treatment of alcoholism: from animal to clinical studies | http://www.ncbi.nlm.nih.gov/pubmed/10075397
- ↑ http://web.archive.org/web/20100116121252/http://www.projectghb.org/laws.htm