Quetiapine: Difference between revisions
>Josikins No edit summary |
>Josikins No edit summary |
||
| Line 1: | Line 1: | ||
Quetiapine (/kwɨˈtaɪ.əpiːn/ kwi-ty-ə-peen) (branded as '''Seroquel''', '''Xeroquel''', '''Ketipinor''') is a short-acting atypical [[antipsychotic]] approved for the treatment of schizophrenia, bipolar disorder, and along with an antidepressant to treat major depressive disorder. | {| class="wikitable" align="right" | ||
! colspan="2" style="background:lightblue;font-size:10pt" | Quetiapine | |||
|- | |||
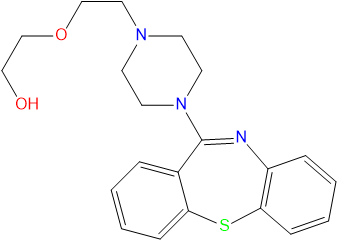
| colspan="2" cellpadding="2" style="text-align:center;font-size:7pt" | [[File:Quetiapine.png |noframe|338px]]<br />[[File:Quetiapine3DanBS.gif|noframe|256px]]<br>The skeletal formula of Quetiapine. | |||
|- | |||
| colspan="2" style="background:lightblue;text-align:center;font-size:9pt" | '''Dosage (oral)''' | |||
|- | |||
| style="font-size:9pt" | ''Threshold'' || style="font-size:9pt" | 10 - 20 mg | |||
|- | |||
| style="font-size:9pt" | ''Light'' || style="font-size:9pt" | 20 - 50 mg | |||
|- | |||
| style="font-size:9pt" | ''Common'' || style="font-size:9pt" | 50 - 150 mg | |||
|- | |||
| style="font-size:9pt" | ''Strong'' || style="font-size:9pt" | 150 - 400 mg | |||
|- | |||
| style="font-size:9pt" | ''Heavy'' || style="font-size:9pt" | 400 mg + | |||
|- | |||
| colspan="2" style="background:lightblue;text-align:center;font-size:9pt" | '''Duration (oral)''' | |||
|- | |||
| style="font-size:9pt" | ''Onset''|| style="font-size:9pt" | 5 - 10 minutes | |||
|- | |||
| style="font-size:9pt" | ''Duration''|| style="font-size:9pt" | 1.5 - 5 hours | |||
|- | |||
| style="font-size:9pt" | ''After effects''|| style="font-size:9pt" | up to 24 hours | |||
|} Quetiapine (/kwɨˈtaɪ.əpiːn/ kwi-ty-ə-peen) (branded as '''Seroquel''', '''Xeroquel''', '''Ketipinor''') is a short-acting atypical [[antipsychotic]] approved for the treatment of schizophrenia, bipolar disorder, and along with an antidepressant to treat major depressive disorder. | |||
Annual sales are approximately $5.7 billion worldwide, with $2.9 billion in the United States.<ref>http://drugpatentwatch.com/ultimate/preview/tradename/index.php?query=SEROQUEL</ref> The U.S. patent,<ref>http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=HITOFF&p=1&u=%2Fnetahtml%2FPTO%2Fsearch-bool.html&r=1&f=G&l=50&co1=AND&d=PTXT&s1=4,879,288.PN.&OS=PN/4,879,288&RS=PN/4,879,288</ref> which was set to expire in 2011, received a pediatric exclusivity extension which pushed its expiration to March 26, 2012.<ref>http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=HITOFF&p=1&u=%2Fnetahtml%2FPTO%2Fsearch-bool.html&r=1&f=G&l=50&co1=AND&d=PTXT&s1=4,879,288.PN.&OS=PN/4,879,288&RS=PN/4,879,288</ref><ref>Blockbuster Drugs That Will Go Generic Soon | http://money.usnews.com/money/blogs/the-best-life/2011/04/29/blockbuster-drugs-that-will-go-generic-soon</ref> The patent has already expired in Canada. Quetiapine was developed by AstraZeneca from 1992-1996 as an improvement from first generation antipsychotics. It was first approved by the FDA in 1997. There are now several generic versions of quetiapine, such as Quepin, Syquel and Ketipinor.<ref>http://www.theodora.com/drugs/quepin_tablets_specifar.html</ref> | Annual sales are approximately $5.7 billion worldwide, with $2.9 billion in the United States.<ref>http://drugpatentwatch.com/ultimate/preview/tradename/index.php?query=SEROQUEL</ref> The U.S. patent,<ref>http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=HITOFF&p=1&u=%2Fnetahtml%2FPTO%2Fsearch-bool.html&r=1&f=G&l=50&co1=AND&d=PTXT&s1=4,879,288.PN.&OS=PN/4,879,288&RS=PN/4,879,288</ref> which was set to expire in 2011, received a pediatric exclusivity extension which pushed its expiration to March 26, 2012.<ref>http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=HITOFF&p=1&u=%2Fnetahtml%2FPTO%2Fsearch-bool.html&r=1&f=G&l=50&co1=AND&d=PTXT&s1=4,879,288.PN.&OS=PN/4,879,288&RS=PN/4,879,288</ref><ref>Blockbuster Drugs That Will Go Generic Soon | http://money.usnews.com/money/blogs/the-best-life/2011/04/29/blockbuster-drugs-that-will-go-generic-soon</ref> The patent has already expired in Canada. Quetiapine was developed by AstraZeneca from 1992-1996 as an improvement from first generation antipsychotics. It was first approved by the FDA in 1997. There are now several generic versions of quetiapine, such as Quepin, Syquel and Ketipinor.<ref>http://www.theodora.com/drugs/quepin_tablets_specifar.html</ref> | ||
Revision as of 14:22, 30 April 2014
Quetiapine (/kwɨˈtaɪ.əpiːn/ kwi-ty-ə-peen) (branded as Seroquel, Xeroquel, Ketipinor) is a short-acting atypical antipsychotic approved for the treatment of schizophrenia, bipolar disorder, and along with an antidepressant to treat major depressive disorder.
Annual sales are approximately $5.7 billion worldwide, with $2.9 billion in the United States.[1] The U.S. patent,[2] which was set to expire in 2011, received a pediatric exclusivity extension which pushed its expiration to March 26, 2012.[3][4] The patent has already expired in Canada. Quetiapine was developed by AstraZeneca from 1992-1996 as an improvement from first generation antipsychotics. It was first approved by the FDA in 1997. There are now several generic versions of quetiapine, such as Quepin, Syquel and Ketipinor.[5]
Chemistry
Pharmocology
Subjective effects
Physical effects
Cognitive effects
Toxicity and harm potential
Tolerance and addiction potential
Interactions
Legal issues
References
- ↑ http://drugpatentwatch.com/ultimate/preview/tradename/index.php?query=SEROQUEL
- ↑ http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=HITOFF&p=1&u=%2Fnetahtml%2FPTO%2Fsearch-bool.html&r=1&f=G&l=50&co1=AND&d=PTXT&s1=4,879,288.PN.&OS=PN/4,879,288&RS=PN/4,879,288
- ↑ http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=HITOFF&p=1&u=%2Fnetahtml%2FPTO%2Fsearch-bool.html&r=1&f=G&l=50&co1=AND&d=PTXT&s1=4,879,288.PN.&OS=PN/4,879,288&RS=PN/4,879,288
- ↑ Blockbuster Drugs That Will Go Generic Soon | http://money.usnews.com/money/blogs/the-best-life/2011/04/29/blockbuster-drugs-that-will-go-generic-soon
- ↑ http://www.theodora.com/drugs/quepin_tablets_specifar.html