Talk:Auditory distortion: Difference between revisions
>Graham Neurological Analysis |
>Graham Asynchrony + Reference |
||
| Line 1: | Line 1: | ||
==Neurological Analysis== | ==Neurological Analysis== | ||
===LSD changes how your brain connects to the thalamus=== | |||
"In addition, LSD-induced RSFC [[https://en.wikipedia.org/wiki/Resting_state_fMRI#Functional Resting State Functional Connectivity]] between the thalamus and right fusiform gyrus and insula correlated with subjective visual and auditory alterations, respectively ([https://doi.org/10.1111/acps.12818 Mueller et al, 2017b]). Remaining to be determined is the way in which LSD-induced increases in thalamocortical connectivity may be linked to the thalamic gating of perceptions ([https://doi.org/10.1111/acps.12818 Mueller et al, 2017b]). In contrast to the higher connectivity between neural networks while under the effects of LSD, LSD globally decreased within-network RSFC (integrity) and within-network signal variance ([http://dx.doi.org/10.1073/pnas.1518377113 Carhart-Harris et al, 2016c]) ([https://psychonautwiki.org/wiki/File:Liechti2017_figure_2a.jpg Figure 2a]). | "In addition, LSD-induced RSFC [[https://en.wikipedia.org/wiki/Resting_state_fMRI#Functional Resting State Functional Connectivity]] between the thalamus and right fusiform gyrus and insula correlated with subjective visual and auditory alterations, respectively ([https://doi.org/10.1111/acps.12818 Mueller et al, 2017b]). Remaining to be determined is the way in which LSD-induced increases in thalamocortical connectivity may be linked to the thalamic gating of perceptions ([https://doi.org/10.1111/acps.12818 Mueller et al, 2017b]). In contrast to the higher connectivity between neural networks while under the effects of LSD, LSD globally decreased within-network RSFC (integrity) and within-network signal variance ([http://dx.doi.org/10.1073/pnas.1518377113 Carhart-Harris et al, 2016c]) ([https://psychonautwiki.org/wiki/File:Liechti2017_figure_2a.jpg Figure 2a]). | ||
[[File:Liechti2017_figure_2a.jpg|400px|thumb|center|Figure 2a]] | [[File:Liechti2017_figure_2a.jpg|400px|thumb|center|Figure 2a]] | ||
(a) Mean percentage differences (+SEM) in CBF (red), integrity (blue), and signal variance (green) in 12 different resting-state networks (RSNs) under LSD relative to placebo (red asterisks indicate statistical significance, *P<0.05; **P<0.01, Bonferroni corrected). (b) Differences in between-RSN RSFC or RSN ‘segregation’ under LSD vs placebo. Each square in the matrix represents the strength of functional connectivity (positive=red, negative=blue) between a pair of different RSNs (parameter estimate values). The matrix on the far right displays the between-condition differences in covariance (t values): red=reduced segregation and blue=increased segregation under LSD. White asterisks represent significant differences (P<0.05, FDR corrected; n=15). Reproduced from [http://dx.doi.org/10.1073/pnas.1518377113 Carhart-Harris et al (2016c)]."<ref>Liechti, M. E. (2017). Modern clinical research on LSD. Neuropsychopharmacology, 42(11), 2114. https://doi.org/10.1038/npp.2017.86</ref> | (a) Mean percentage differences (+SEM) in CBF (red), integrity (blue), and signal variance (green) in 12 different resting-state networks (RSNs) under LSD relative to placebo (red asterisks indicate statistical significance, *P<0.05; **P<0.01, Bonferroni corrected). (b) Differences in between-RSN RSFC or RSN ‘segregation’ under LSD vs placebo. Each square in the matrix represents the strength of functional connectivity (positive=red, negative=blue) between a pair of different RSNs (parameter estimate values). The matrix on the far right displays the between-condition differences in covariance (t values): red=reduced segregation and blue=increased segregation under LSD. White asterisks represent significant differences (P<0.05, FDR corrected; n=15). Reproduced from [http://dx.doi.org/10.1073/pnas.1518377113 Carhart-Harris et al (2016c)]."<ref>Liechti, M. E. (2017). Modern clinical research on LSD. Neuropsychopharmacology, 42(11), 2114. https://doi.org/10.1038/npp.2017.86</ref> | ||
===Hallucinogen distortions are from disrupted synchrony of your neurons=== | |||
pg 634 "Hallucinogens disrupt PFC network activity, and after administration of hallucinogens, individual pyramidal neurons are less likely to fire in synchrony with other pyramidal neurons, and low-frequency network activity is attenuated. Since the efficiency of cortical information processing is dependent on cortical synchrony, these effects could markedly alter how the PFC functions. Furthermore, the increase in recurrent cortical network activity induced by hallucinogens could compete with responses induced by sensory input, potentially altering sensory processing. Since pyramidal neurons in PFC layer V are the source of projections to other cortical and subcortical structures, hallucinogen effects on PFC activity and processing could profoundly affect how the PFC regulates activity in those projection sites. For example, systemic administration of DOI to rats increases the output of mPFC neurons projecting to DRN and the VTA in the midbrain, which give rise to serotonergic and dopaminergic projections, respectively. It has been proposed that one of the major effects of hallucinogens may be to alter the function of cortico-striato-thalamic pathways (CSTC loops) that regulate the gating of cortical information processing. Altered activity in these circuits, due to the direct and indirect effects of hallucinogens on their cortical and subcortical components, could produce gating deficits that would result in sensory overload, hallucinations, and disruptions of normal cognitive processes. Indeed, one of the known effects of hallucinogens in humans and rodents is disruption of measures of sensorimotor gating such as prepulse inhibition. The hypothesis that hallucinogens act by disrupting CSTC feedback loops has received some support from imaging studies showing that psilocybin alters activity in cortical and subcortical components of the “limbic” CSTC loop."<ref>Halberstadt, A. L., & Geyer, M. A. (2013). Neuropharmacology of lysergic acid diethylamide (LSD) and other hallucinogens. In Biological research on addiction (pp. 625-635). https://doi.org/10.1016/B978-0-12-398335-0.00061-3</ref> | |||
==References== | ==References== | ||
<references/> | <references/> | ||
Revision as of 00:30, 3 March 2018
Neurological Analysis
LSD changes how your brain connects to the thalamus
"In addition, LSD-induced RSFC [Resting State Functional Connectivity] between the thalamus and right fusiform gyrus and insula correlated with subjective visual and auditory alterations, respectively (Mueller et al, 2017b). Remaining to be determined is the way in which LSD-induced increases in thalamocortical connectivity may be linked to the thalamic gating of perceptions (Mueller et al, 2017b). In contrast to the higher connectivity between neural networks while under the effects of LSD, LSD globally decreased within-network RSFC (integrity) and within-network signal variance (Carhart-Harris et al, 2016c) (Figure 2a).
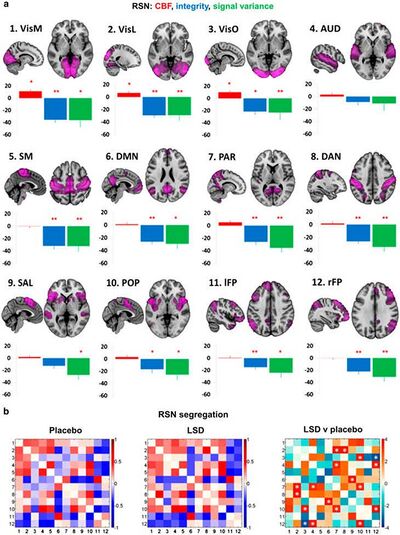
(a) Mean percentage differences (+SEM) in CBF (red), integrity (blue), and signal variance (green) in 12 different resting-state networks (RSNs) under LSD relative to placebo (red asterisks indicate statistical significance, *P<0.05; **P<0.01, Bonferroni corrected). (b) Differences in between-RSN RSFC or RSN ‘segregation’ under LSD vs placebo. Each square in the matrix represents the strength of functional connectivity (positive=red, negative=blue) between a pair of different RSNs (parameter estimate values). The matrix on the far right displays the between-condition differences in covariance (t values): red=reduced segregation and blue=increased segregation under LSD. White asterisks represent significant differences (P<0.05, FDR corrected; n=15). Reproduced from Carhart-Harris et al (2016c)."[1]
Hallucinogen distortions are from disrupted synchrony of your neurons
pg 634 "Hallucinogens disrupt PFC network activity, and after administration of hallucinogens, individual pyramidal neurons are less likely to fire in synchrony with other pyramidal neurons, and low-frequency network activity is attenuated. Since the efficiency of cortical information processing is dependent on cortical synchrony, these effects could markedly alter how the PFC functions. Furthermore, the increase in recurrent cortical network activity induced by hallucinogens could compete with responses induced by sensory input, potentially altering sensory processing. Since pyramidal neurons in PFC layer V are the source of projections to other cortical and subcortical structures, hallucinogen effects on PFC activity and processing could profoundly affect how the PFC regulates activity in those projection sites. For example, systemic administration of DOI to rats increases the output of mPFC neurons projecting to DRN and the VTA in the midbrain, which give rise to serotonergic and dopaminergic projections, respectively. It has been proposed that one of the major effects of hallucinogens may be to alter the function of cortico-striato-thalamic pathways (CSTC loops) that regulate the gating of cortical information processing. Altered activity in these circuits, due to the direct and indirect effects of hallucinogens on their cortical and subcortical components, could produce gating deficits that would result in sensory overload, hallucinations, and disruptions of normal cognitive processes. Indeed, one of the known effects of hallucinogens in humans and rodents is disruption of measures of sensorimotor gating such as prepulse inhibition. The hypothesis that hallucinogens act by disrupting CSTC feedback loops has received some support from imaging studies showing that psilocybin alters activity in cortical and subcortical components of the “limbic” CSTC loop."[2]
References
- ↑ Liechti, M. E. (2017). Modern clinical research on LSD. Neuropsychopharmacology, 42(11), 2114. https://doi.org/10.1038/npp.2017.86
- ↑ Halberstadt, A. L., & Geyer, M. A. (2013). Neuropharmacology of lysergic acid diethylamide (LSD) and other hallucinogens. In Biological research on addiction (pp. 625-635). https://doi.org/10.1016/B978-0-12-398335-0.00061-3