This is an unofficial archive of PsychonautWiki as of 2025-08-11T15:14:44Z. Content on this page may be outdated, incomplete, or inaccurate. Please refer to the original page for the most up-to-date information.
Substituted phenidates: Difference between revisions
Jump to navigation
Jump to search
>Dextromethorphan |
>BronzeManul →List of substituted phenidates: Added 4-Methyl Methylphenidate |
||
| Line 21: | Line 21: | ||
|- | |- | ||
| [[Propylphenidate]] || H || H || CH<sub>2</sub>CH<sub>2</sub>CH<sub>3</sub> || [[File:Propylphenidate.png|170px]] | | [[Propylphenidate]] || H || H || CH<sub>2</sub>CH<sub>2</sub>CH<sub>3</sub> || [[File:Propylphenidate.png|170px]] | ||
|- | |||
| [[4-Methyl Methylphenidate]] || H || CH<sub>3</sub> || CH<sub>3</sub> || [[File:4Me-MPH.png|171px]] | |||
|- | |- | ||
| [[3,4-CTMP]] || Cl || Cl || CH<sub>3</sub> || [[File:34-ctmp.png|171px]] | | [[3,4-CTMP]] || Cl || Cl || CH<sub>3</sub> || [[File:34-ctmp.png|171px]] | ||
Revision as of 00:02, 26 October 2016
Substituted phenidates, also known as phenidates, are a class of chemicals that include compounds with psychoactive effects.
Chemistry
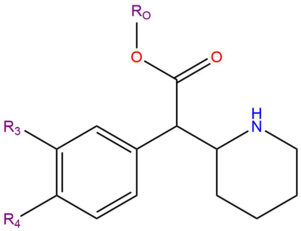
Substituted phenidates are a chemical class based upon the molecule Methylphenidate. Methylphenidate is made up of a phenethylamine molecule with a carbon chain substitution at the Rα position that links to the RN position forming a piperidine ring, and a substitution at the Rβ position of methyl acetate.
List of substituted phenidates
| Compound | R3 | R4 | RO | Structure |
|---|---|---|---|---|
| Methylphenidate | H | H | CH3 | 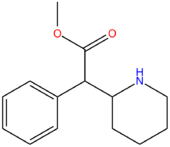
|
| Ethylphenidate | H | H | CH2CH3 | 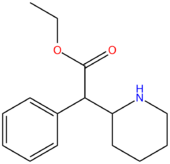
|
| Isopropylphenidate | H | H | CH(CH3)2 | 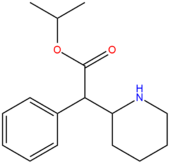
|
| Propylphenidate | H | H | CH2CH2CH3 | 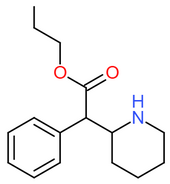
|
| 4-Methyl Methylphenidate | H | CH3 | CH3 | 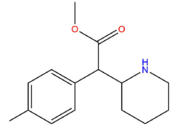
|
| 3,4-CTMP | Cl | Cl | CH3 | 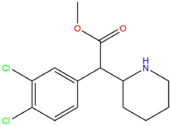
|
| 4F-MPH | H | F | CH3 | 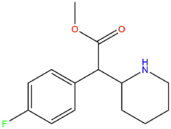
|
| 4F-EPH | H | F | CH2CH3 | 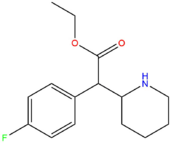
|
| Methylnaphthidate | CH=CH- | CH=CH- | CH3 | 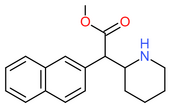
|
| Ethylnaphthidate | CH=CH- | CH=CH- | CH2CH3 | 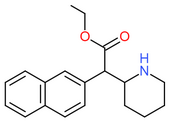
|