Volumetric liquid dosing
 |
This guide is provided for informational and educational purposes only. We do not encourage you to break the law and cannot claim any responsibility for your actions. |
Volumetric dosing is the process of dissolving a compound in a liquid to make it easier to measure. In the interest of harm reduction, it is essential to prepare certain compounds which are too potent to measure with traditional weighing scales within a liquid solution.
Many psychoactive substances, including benzodiazepines and certain psychedelics, are active at less than a single milligram. Such small quantities cannot be accurately measured with common digital scales, so the drug must instead be dosed volumetrically by weighing out larger amounts the compound and dissolving it in a calculated volume of a suitable liquid.
Solvent
Certain psychoactive substances (particularly benzodiazepines) are practically insoluble in water, but will dissolve at various concentrations in other easy to acquire solvents. These include:
- Ethanol
- Propylene glycol
- Glycerine
The first important consideration is the maximum concentration at which your chosen solvent will dissolve your product. A value can usually be found with minimal effort on the internet, using a search term like "<material> <solvent> solubility".
Beneficially, ethanol is miscible with most solvents. Just a few drops is usually enough to increase the maximum concentration dramatically, even when using water as the major component.
Measurement
Liquid
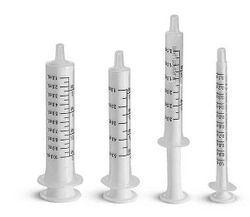
For best accuracy, an oral syringe should be used to measure the calculated volume of liquid. For efficient dosing, a 1ml syringe with 0.1ml graduations can be used with solutions at a concentration of 10mg/1ml; this provides 1mg/0.1ml graduation. It may be better to use a lower concentration if your usual dose is below 1mg.
Powder
Preferably a fresh batch of known weight would be used, but it's best to weigh the entirety of the material before dissolution, so you can calculate the necessary volume of solvent required to achieve the desired concentration.
For example; to achieve 10mg/1ml, measure the weight of material in milligrams, then divide the value by 10. So for 1000mg material, 100ml of solvent will be required.
Dissolution
After weighing the material, calculating the concentration, and measuring the required volume of solvent; using a suitable container add the liquid then carefully mix in the compound. Seal tightly and mix thoroughly by shaking the container vigorously.
To aid in dissolution, the container can be placed in a heat bath to warm it up. After a while the solvent will become less viscous and can be mixed more thoroughly.
Storage
- Avoid exposure to direct sunlight or heat
- Label the container with contents and concentration
- Keep in a safe place away from children