Salting out isopropyl alcohol: Difference between revisions
>Corticosteroid Highlighting that the salt must not be iodized (I actually missed this because I read over it too fast) with an explanation as to why. |
>Corticosteroid Highlighting that the salt must not be iodized (I actually missed this because I read over it too fast) with an explanation as to why. |
(No difference)
| |
Revision as of 01:15, 18 September 2017
 |
This guide is provided for informational and educational purposes only. We do not encourage you to break the law and cannot claim any responsibility for your actions. |
Salting out isopropyl alcohol increases the potency by using salt to draw the water out of the alcohol. The salt dissolves in the water but is insoluble in isopropyl alcohol, making the water heavier and separate to the bottom while the isopropyl alcohol floats on top.
Risks and hazards
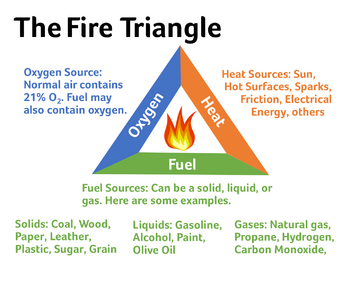
Be incredibly careful when working with isopropyl alcohol or any other flammable substances. High temperatures, sparks, or open flames can cause fires or explosions since isopropyl alcohol emits flammable vapors! Breathing isopropyl alcohol vapors is dangerous and these experiments should be done in a well-ventilated area with proper protective equipment.
In order for an explosive event to occur there needs to be an ignition source or spark. This spark can come from common sense items such as a lighter or pilot light on a stove, but sparks can also occur in less obvious ways such as static electricity , certain materials grinding against eachother, and electric components found on stoves or car components[1]. Electric sparks can be created simply by wearing clothes - the common "shock" one experiences after shuffling around with socks on and touching a metal object, along with the sparks created by the compressors in most common kitchen freezers, is sufficient to cause an explosive event.
The flammability of a gas depends on its concentration in the atmosphere. If the concentration of isopropyl alcohol falls in this range and there is a spark present, there will be an ignition and an explosive event[2].
Materials
- Isopropyl alcohol - the vapors resulting from evaporating this liquid are highly flammable.

- Non-iodized salt. The salt must not contain iodine. Sodium chloride is not soluble in isopropyl alcohol, meanwhile iodine is. This threatens the purity of the end product. Making it worse, the iodine is not very soluble at all in water.
- Siphon (syringe, turkey baster, pipette)
- Large resealable glass jar
Steps
Step 1: Add salt to the jar
Salt is poured into the jar until it is about 1/4 full.
Step 2: Add the isopropyl alcohol
Isopropyl alcohol is slowly poured into the jar until the jar is filled to the 3/4 mark and then sealed.
Step 3: Mixing
The jar is shaken thoroughly for at least 5 minutes.
Step 4: Let it settle
The jar is then placed down and left undisturbed for approximately a half hour to let the alcohol and salt water separate.
Step 5: Siphon off the isopropyl alcohol
The jar is opened and the clear liquid at the top is the anhydrous isopropyl alcohol. It is siphoned off and placed in an airtight container for storage. No salt should be siphoned and a thin layer of isopropyl alcohol should be left in the jar to avoid any salt being drawn up. Be sure to perform this step in a well ventilated area to minimize the risks of explosions due to the build up of isopropyl alcohol vapors.
Step 6: Salt water
The salt water can be disposed of as it is no longer useful. The remaining isopropyl alcohol in the jar is to be discarded as well.
References
- ↑ "Fire Protection and Prevention", OSHA https://www.osha.gov/dte/grant_materials/fy09/sh-18796-09/fireprotection.pdf
- ↑ Flammability of Gasses http://people.clarkson.edu/~wwilcox/Design/flamlim2.pdf