DOM: Difference between revisions
>Josikins |
>Josikins No edit summary |
||
| Line 33: | Line 33: | ||
'''2,5-Dimethoxy-4-methylamphetamine''' ('''DOM''') or '''STP''' is a [[psychedelics|psychedelic drug]] of the [[Phenethylamines|substituted phenethylamine]] and [[Amphetamines|substituted amphetamine]] chemical classes. It is a member of the [[DOx]] family of compounds. | '''2,5-Dimethoxy-4-methylamphetamine''' ('''DOM''') or '''STP''' is a [[psychedelics|psychedelic drug]] of the [[Phenethylamines|substituted phenethylamine]] and [[Amphetamines|substituted amphetamine]] chemical classes. It is a member of the [[DOx]] family of compounds. | ||
DOM was first synthesized and tested in 1963 by Alexander Shulgin, who was investigating the effect of 4-position substitutions on psychedelic amphetamines.<ref>Shulgin, Alexander (1991). Pihkal : a chemical love story. Berkeley, CA: Transform Press. pp. 53–56.</ref> It has very limited history of human usage prior to the 1991 publication of its synthesis and pharmacology in the ''[[PiHKAL]]'' (''Phenethylamines i Have Known And Loved'').<ref name="PiHKAL">http://www.erowid.org/library/books_online/pihkal/pihkal.shtml</ref> by [[Alexander Shulgin]]. In modern times it is used as a recreational drug and an entheogen, rarely sold on the streets and almost exclusively obtained as a grey area research chemical through the use of online vendors. | |||
DOM is a highly dose sensitive psychedelic that is often sold on blotting paper and known for its strong visuals and intense body load. Many reports also indicate that the effects of this chemical may be overly intense for those who are not already experienced with psychedelics. | DOM is a highly dose sensitive psychedelic that is often sold on blotting paper and known for its strong visuals and intense body load. Many reports also indicate that the effects of this chemical may be overly intense for those who are not already experienced with psychedelics. | ||
| Line 42: | Line 42: | ||
==Pharmacology== | ==Pharmacology== | ||
DOM | DOM is a selective [[5-HT2A receptor|5-HT<sub>2A</sub>]], [[5-HT2B receptor|5-HT<sub>2B</sub>]], and [[5-HT2C receptor|5-HT<sub>2C</sub> receptor]] [[partial agonist]]. Its psychedelic effects are mediated by its [[agonist]]ic properties at the 5-HT<sub>2A</sub> receptor. Due to its selectivity, DOM is often used in scientific research when studying the [[5-HT2|5-HT<sub>2</sub> receptor]] subfamily. DOM is a [[Chirality (chemistry)|chiral]] molecule, and ''R''-(-)-DOM is the more active [[enantiomer]], functioning as a potent agonist of the serotonin [[5-HT]] family of receptors; mainly of the [[5-HT2]] subtype.<ref>Sanders-Bush, Burris, KD; Knoth, K, (September 1988). "Lysergic acid diethylamide and 2,5-dimethoxy-4-methylamphetamine are partial agonists at serotonin receptors linked to phosphoinositide hydrolysis" Journal of Pharmacology and Experimental Therapeutics '''246''' 3 924–928</ref> | ||
=Subjective effects= | =Subjective effects= | ||
Revision as of 21:01, 28 February 2014
2,5-Dimethoxy-4-methylamphetamine (DOM) or STP is a psychedelic drug of the substituted phenethylamine and substituted amphetamine chemical classes. It is a member of the DOx family of compounds.
DOM was first synthesized and tested in 1963 by Alexander Shulgin, who was investigating the effect of 4-position substitutions on psychedelic amphetamines.[1] It has very limited history of human usage prior to the 1991 publication of its synthesis and pharmacology in the PiHKAL (Phenethylamines i Have Known And Loved).[2] by Alexander Shulgin. In modern times it is used as a recreational drug and an entheogen, rarely sold on the streets and almost exclusively obtained as a grey area research chemical through the use of online vendors.
DOM is a highly dose sensitive psychedelic that is often sold on blotting paper and known for its strong visuals and intense body load. Many reports also indicate that the effects of this chemical may be overly intense for those who are not already experienced with psychedelics.
Chemistry
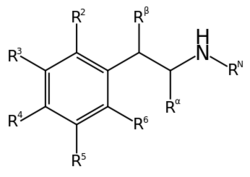
DOM is a substituted alpha-methylated phenethylamine, a class of compounds commonly known as amphetamines. [3] The phenethylamine equivalent (lacking the alpha-methyl group) is 2C-D.
Pharmacology
DOM is a selective 5-HT2A, 5-HT2B, and 5-HT2C receptor partial agonist. Its psychedelic effects are mediated by its agonistic properties at the 5-HT2A receptor. Due to its selectivity, DOM is often used in scientific research when studying the 5-HT2 receptor subfamily. DOM is a chiral molecule, and R-(-)-DOM is the more active enantiomer, functioning as a potent agonist of the serotonin 5-HT family of receptors; mainly of the 5-HT2 subtype.[4]
Subjective effects
The physical effects of DOM can be broken down into six components all of which progressively intensify proportional to dosage. These are described below and generally include:
- Spontaneous tactile sensations - the body high of DOM is manifested as somewhat intense in comparison to most classical psychedelics, however in comparison to DOC and the overwhelming forcefulness of 2C-E, it can actually be considered quite mild. The sensation itself can be described as a constantly present yet somewhat mild energetic pins and needles sensation that encompasses a person’s entire body. This is coupled with a euphoric, fast moving, sharp and location specific tingling sensation. It is usually felt over every square inch of the skin but occasionally manifests itself in the form of a continuously shifting tingling sensation that travels up and down the body in spontaneous waves.
- Stimulation - in terms of its effects on the physical energy levels of the tripper DOM is usually considered to be extremely stimulating at levels which do not become overwhelming, resulting in a shakiness and unsteadiness of the hands but encouraging trippers to move around, run, dance, climb and generally engage in physical activities. In comparison, other more commonly used psychedelics such as psilocin are generally sedating and relaxed.
- Increased bodily control
- Enhancement of touch - feelings of enhanced tactile sensation are consistently present at moderate levels throughout most DOM trips. Once Level 7A visuals are reached, an intense sensation of suddenly becoming aware of and being able to feel every single nerve ending across a person's entire body all at once is consistently present.
- Nausea - mild to extreme nausea are reported when consumed in moderate to high dosages and either passes once the tripper has vomited or gradually fades by itself as the peak sets in.
- Vasoconstriction - this effect is usually only present at higher dosages but can be particularly uncomfortable.
The head space of DOM is described by many as one of extreme mental stimulation and a powerful enhancement of a person's current mental state.
The total sum of these cognitive components regardless of the setting generally includes:
- Introspection - this component is consistently manifested only in the context of a non social setting in which the user is alone.
- Increased empathy, love and sociability - this component is consistently manifested only in the context of social settings in which one is within the company of others. These feelings of sociability, love and empathy are a little weaker and less sharp than those found on substances such as MDMA and 2C-B but still prove strong enough to provide long lasting therapeutic effects.
- Acceleration of thought
- Time distortion
- Feelings of fascination, importance and awe
- Conceptual thinking
- Connectivity of thought
- Enhancement of current mind state
- Removal of cultural filter
- Ego suppression, loss and death
DOM presents a full and complete array of possible visual enhancements which generally includes:
As for visual distortions and alterations, the effects experienced are detailed below:
- Drifting (Melting, Flowing, Breathing and morphing) - In comparison to other psychedelics this effect can be described as highly detailed, slow and smooth in motion, static in their appearance and unrealistic/ cartoon like in style.
- Tracers
- After images
- Texture repetition
- Colour shifting
- Scenery slicing
The visual geometry that is present throughout this trip can be described as more similar in appearance to that of 4-AcO-DMT, Ayahuasca than that of LSD, 2C-B or 2C-I. They can be comprehensively described as structured in their organization, organic in geometric style, intricate in complexity, large in size, fast and smooth in motion, colourful in scheme, glossy in color, sharp in their edges and equally rounded and angular in their corners. They give off a contradictory natural and synthetic feel to them that at higher dosages are significantly more likely to result in states of Level 7B visual geometry over Level 7A.
DOM and other substituted amphetamines produce a full range of high level hallucinatory states in a fashion that is more consistent and reproducible than that of many other commonly used psychedelics. This holds particularly true in comparison to other substances within the phenethylamine family. These effects include:
- External hallucinations
- Internal hallucinations - in comparison to other psychedelics such as LSD, DOM is extremely high in imagery embedded within visual geometry. This particular effect commonly contains hallucinations with with scenarios, settings, concepts and autonomous entity contact. They are more common within dark environments and can be described as internal in their manifestation, lucid in believability, interactive in style and almost exclusively of religious, spiritual, mystical or a transcendental nature in their overall theme.
The auditory effects of DOM are common in their occurrence and exhibit a full range of effects which commonly includes:
Toxicity and Harm Potential
Lethal Dosage
The toxicity and long term health effects of recreational DOM has limited research in a scientific context. This is because DOM is a research chemical with very little history of human usage. Anecdotal evidence from people who have tried DOM within the psychedelic community suggests that there are no negative health effects attributed to simply trying this drug at low to moderate doses or using it very sparingly (but nothing can be completely guaranteed).
Tolerance and Addiction Potential
DOM is not physically addictive and many users experience a frequency self-regulating quality to the drug. Tolerance for DOM seems to be very mild and does not build up without repeated use over a short period of time.
Legal Issues
- UK: DOM is a Class A drug.
- USA: DOM is a Schedule I drug.
- Canada: DOM is a Schedule I drug.
- New Zealand: DOM is a Class C drug.
- Belgium: DOM is a Schedule I drug.
References
- ↑ Shulgin, Alexander (1991). Pihkal : a chemical love story. Berkeley, CA: Transform Press. pp. 53–56.
- ↑ http://www.erowid.org/library/books_online/pihkal/pihkal.shtml
- ↑ http://books.google.com/books?id=gvanBNhSQ5YC&pg=PA157&lpg=PA157&dq=DOM+is+a+substituted+alpha-methylated+phenethylamine,&source=bl&ots=QBm-rgNHFs&sig=cOGzSnEkZP--IEpEJZQLldyoxwc&hl=en&sa=X&ei=gIfUUrL3IbPRsASMnIH4Cw&ved=0CF4Q6AEwBg#v=onepage&q=DOM%20is%20a%20substituted%20alpha-methylated%20phenethylamine%2C&f=false
- ↑ Sanders-Bush, Burris, KD; Knoth, K, (September 1988). "Lysergic acid diethylamide and 2,5-dimethoxy-4-methylamphetamine are partial agonists at serotonin receptors linked to phosphoinositide hydrolysis" Journal of Pharmacology and Experimental Therapeutics 246 3 924–928
