Methylone: Difference between revisions
>Josikins No edit summary |
>Josikins No edit summary |
||
| Line 1: | Line 1: | ||
{{Stub}} | {{Stub}} | ||
{{Substance | |||
|name=BK-MDMA | |||
|abbreviation= | |||
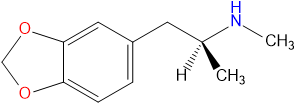
|IUPAC=(RS)-1-(benzo[d][1,3]dioxol-5-yl)-N-methylpropan-2-amine | |||
|molar_mass=193.242 | |||
|chemspider=1556 | |||
|erowid=mdma | |||
|img-skel=File:MDMA2.png | |||
|img-skel-width=295px | |||
|img-3d= | |||
|dosage-method= | |||
|dosage-threshold=60mg | |||
|dosage-light=100 - 150mg | |||
|dosage-common=150 - 250mg | |||
|dosage-strong=200 - 300mg | |||
|dosage-heavy=300mg + | |||
|duration-total= | |||
|duration-onset=20 - 70 minutes | |||
|duration-coming-up=10 - 15 minutes | |||
|duration-peak=2 - 4 hours | |||
|duration-coming-down= 1 hour | |||
|duration-after-effects=12 hours | |||
}} | |||
'''Methylone''', also known as '''M1''', '''3,4-methylenedioxy-N-methylcathinone''', '''MDMC''', '''bk-MDMA''' is an [[entactogen]] and [[stimulant]] of the [[phenethylamine]], [[amphetamine]] and [[cathinone]] classes. It was first synthesized by chemists Peyton Jacob III and [[Alexander Shulgin]] in 1996 for potential use as an antidepressant.<ref>http://worldwide.espacenet.com/textdoc?DB=EPODOC&IDX=WO9639133</ref> Methylone is a close structural analog of [[MDMA]], differing by the addition of a β-ketone group.<ref>Inhibition of plasma membrane monoamine transporters by beta-ketoamphetamines | http://www.ncbi.nlm.nih.gov/pubmed/10528135</ref> | '''Methylone''', also known as '''M1''', '''3,4-methylenedioxy-N-methylcathinone''', '''MDMC''', '''bk-MDMA''' is an [[entactogen]] and [[stimulant]] of the [[phenethylamine]], [[amphetamine]] and [[cathinone]] classes. It was first synthesized by chemists Peyton Jacob III and [[Alexander Shulgin]] in 1996 for potential use as an antidepressant.<ref>http://worldwide.espacenet.com/textdoc?DB=EPODOC&IDX=WO9639133</ref> Methylone is a close structural analog of [[MDMA]], differing by the addition of a β-ketone group.<ref>Inhibition of plasma membrane monoamine transporters by beta-ketoamphetamines | http://www.ncbi.nlm.nih.gov/pubmed/10528135</ref> | ||
Revision as of 10:22, 24 October 2014
 |
This article is a stub. As such, it may contain incomplete or wrong information. You can help by expanding it. |
Methylone, also known as M1, 3,4-methylenedioxy-N-methylcathinone, MDMC, bk-MDMA is an entactogen and stimulant of the phenethylamine, amphetamine and cathinone classes. It was first synthesized by chemists Peyton Jacob III and Alexander Shulgin in 1996 for potential use as an antidepressant.[1] Methylone is a close structural analog of MDMA, differing by the addition of a β-ketone group.[2]
Methylone is often used as a substitute for MDMA due to its similar range of subjective effects. However, in spite of behavioral and pharmacological similarities between methylone and MDMA, the observed subjective effects of both drugs are not completely identical. Alexander Shulgin wrote of the former:_Ask_Dr._Shulgin_Online-3|[3]
"[Methylone] has almost the same potency of MDMA, but it does not produce the same effects. It has an almost antidepressant action, pleasant and positive, but not the unique magic of MDMA."
Toxicity and Harm Potential
Chemistry
Methylone, also known as 3,4-methylenedioxy-N-methylamphetamine, is part of the phenethylamine and amphetamine families. There is a methylenedioxy ring attached to carbons R3 and R4, as well as methyl chains at Rα and RN.
Pharmacology
Methylone acts as a mixed reuptake inhibitor/releasing agent of serotonin, norepinephrine, and dopamine.[3][10] These are the neurotransmitters in charge of pleasure, motivation and focus. This is done by inhibiting the reuptake and reabsorption of the neurotransmitters after they have performed their function of transmitting a neural impulse, essentially allowing them to accumulate and be reused, causing physically stimulating and euphoric effects.
In comparison to MDMA, it has approximately 3x lower affinity for the serotonin transporter, while its affinity for the norepinephrine and dopamine transporters is similar.[3][10] Notably, methylone's affinity for the vesicular monoamine transporter 2 (VMAT2) is about 13x lower than that of MDMA.[3] The results of these differences in pharmacology relative to MDMA are that methylone is less potent in terms of dose, has more balanced catecholaminergic effects relative to serotonergic, and behaves more like a reuptake inhibitor like methylphenidate than a releaser like amphetamine; however, methylone still has relatively robust releasing capabilities.[10]
Subjective effects
Physical effects
Cognitive effects
Visual effects
Legal issues
- Netherlands: In the Netherlands, methylone is covered under the medicine act. Because methylone is not registered officially, as such, it is forbidden to trade in methylone.[4]
- New Zealand: In New Zealand, although methylone is not explicitly scheduled and falls outside the strict definitions of an "amphetamine analogue" in the Misuse of Drugs Act, it is considered to be "substantially similar" to methcathinone and is thus considered by law enforcement authorities to be a Class C illegal drug.
- United Kingdom: In the UK, methylone is illegal since the 16/04/2010 revision of the misuse of drugs act.
- Sweden: Methylone has been listed as a schedule I narcotics in Sweden as of Oct 1.[5]
- Canada: Although not listed as a Schedule 1[6] substance, Health Canada reports that methylone falls under the scheduling as an analogue of amphetamine. However, Methylone bears the exact chemical difference between amphetamine and cathinone - and cathinone is listed as not being an analogue of amphetamine. Leading to imply that methylone is unscheduled in Canada.[7]
- United States: As of October 21, 2011 the DEA has issued an emergency ban on methylone. It is illegal to possess and distribute.[8][9]
Etymology
"Methylone" is also a trademarked brand name for an injectable form of methylprednisolone, a corticosteroid hormone used to treat arthritis and severe allergic reactions; hence, methylone may be confused with it. Aside from context, they can be distinguished by the fact that the name will usually be capitalized when referring to the prescription drug.
A proposed alternate name is bk-MDMA, or beta-keto-MDMA. While this nomenclature has not caught on because the name "methylone" became widely used before the conflicting Methylone trademark was noticed, the analogous names for related chemicals bk-MDEA and bk-MBDB have become the established names for those substances.
See Also
References
- ↑ http://worldwide.espacenet.com/textdoc?DB=EPODOC&IDX=WO9639133
- ↑ Inhibition of plasma membrane monoamine transporters by beta-ketoamphetamines | http://www.ncbi.nlm.nih.gov/pubmed/10528135
- _Ask_Dr._Shulgin_Online_3-0|↑ "Cathinone | Ask Dr. Shulgin Online".
- ↑ van Amsterdam et al., 2004
- ↑ http://www.lakemedelsverket.se/upload/lvfs/LVFS_2010_23.pdf
- ↑ http://isomerdesign.com/Cdsa/schedule.php?schedule=1§ion=18.5&structure=C
- ↑ http://isomerdesign.com/Cdsa/definitions.php?structure=C
- ↑ "Chemicals Used in 'Bath Salts' Now Under Federal Control and Regulation". USA Dept of Justice. Retrieved 22 April 2014. | http://www.justice.gov/dea/divisions/hq/2011/hq102111.shtml
- ↑ "Schedules of Controlled Substances: Placement of Methylone Into Schedule I". Retrieved 22 April 2014. | http://www.deadiversion.usdoj.gov/fed_regs/rules/2012/fr1017.htm