Adamantanes: Difference between revisions
>Tracer m Grammatics |
>Tracer m Grammatics |
||
| Line 13: | Line 13: | ||
==Pharmacology== | ==Pharmacology== | ||
The psychotropic activity spectrum of many adamantane-containing compounds is determined by the nature of their amine substituents, their positions in the polycyctic nucleus, and the basicity of the amino groups. Many have been demonstrated to possesses central dopaminergic psychostimulant-like properties. Some adamantanes produce blocking of the open channels of NMDA and | The psychotropic activity spectrum of many adamantane-containing compounds is determined by the nature of their amine substituents, their positions in the polycyctic nucleus, and the basicity of the amino groups. Many have been demonstrated to possesses central dopaminergic psychostimulant-like properties. Some adamantanes produce blocking of the open channels of NMDA and n-cholinergic receptors. It was found that some the derivatives of adamantane markedly increases the production of [[acetylcholine]] in the frontal cortex and hippocampus.<ref name="Adamantane Pharmacology"/> | ||
In 2004, it was discovered that amantadine and [[memantine]] bind to and act as [[agonists]] of the σ1 [[receptor]] (Ki = 7.44 µM and 2.60 µM, respectively), and that activation of the σ1 receptor is involved in the central [[dopaminergic]] effects of [[wikipedia:amantadine|amantadine]] at therapeutically relevant concentrations. These findings may also extend to the other adamantanes such as [[wikipedia:Adapromine|adapromine]], [[wikipedia:Rimantadine|rimantadine]], and [[bromantane]], and might potentially explain the psychostimulant-like effects of this family of compounds, possibly including those of bromantane.<ref>Involvement of the sigma 1 receptor in the modulation of dopaminergic transmission by amantadine. (PubMed.gov / NCBI) | https://www.ncbi.nlm.nih.gov/pubmed/15090047</ref> | In 2004, it was discovered that amantadine and [[memantine]] bind to and act as [[agonists]] of the σ1 [[receptor]] (Ki = 7.44 µM and 2.60 µM, respectively), and that activation of the σ1 receptor is involved in the central [[dopaminergic]] effects of [[wikipedia:amantadine|amantadine]] at therapeutically relevant concentrations. These findings may also extend to the other adamantanes such as [[wikipedia:Adapromine|adapromine]], [[wikipedia:Rimantadine|rimantadine]], and [[bromantane]], and might potentially explain the psychostimulant-like effects of this family of compounds, possibly including those of bromantane.<ref>Involvement of the sigma 1 receptor in the modulation of dopaminergic transmission by amantadine. (PubMed.gov / NCBI) | https://www.ncbi.nlm.nih.gov/pubmed/15090047</ref> | ||
Revision as of 23:47, 5 June 2019
 |
This article is a stub. As such, it may contain incomplete or wrong information. You can help by expanding it. |
Adamantanes refers to a class of compounds which typically act as dopaminergic stimulants and NMDA receptor antagonists.
History
In the 1960s, the adamantane derivative amantadine was developed as an antiviral drug for the treatment of influenza. Other adamantane antivirals subsequently followed, such as rimantadine and adapromine.[1] It was serendipitously discovered in 1969 that amantadine possesses central dopaminergic psychostimulant-like properties,[2] and subsequent investigation revealed that rimantadine and adapromine also possess such properties.[3] Amantadine was then developed and introduced for the treatment of Parkinson's disease due to its ability to increase dopamine levels in the brain. It has also notably since been used to help alleviate fatigue in multiple sclerosis. With the knowledge of the dopaminergic psychostimulant effects of the adamantane derivatives, in the 1980s was developed bromantane as "a drug having psychoactivating and adaptogen properties under complicated conditions".[4]
Adamantane was identified as a key structural subunit in several synthetic cannabinoid designer drugs, namely AB-001 and SDB-001.[5]
Chemistry
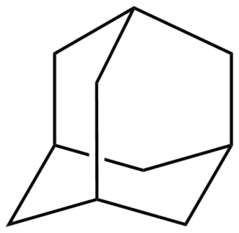
Adamantane molecules consists of three connected cyclohexane rings arranged in the "armchair" configuration.
Pharmacology
The psychotropic activity spectrum of many adamantane-containing compounds is determined by the nature of their amine substituents, their positions in the polycyctic nucleus, and the basicity of the amino groups. Many have been demonstrated to possesses central dopaminergic psychostimulant-like properties. Some adamantanes produce blocking of the open channels of NMDA and n-cholinergic receptors. It was found that some the derivatives of adamantane markedly increases the production of acetylcholine in the frontal cortex and hippocampus.[3]
In 2004, it was discovered that amantadine and memantine bind to and act as agonists of the σ1 receptor (Ki = 7.44 µM and 2.60 µM, respectively), and that activation of the σ1 receptor is involved in the central dopaminergic effects of amantadine at therapeutically relevant concentrations. These findings may also extend to the other adamantanes such as adapromine, rimantadine, and bromantane, and might potentially explain the psychostimulant-like effects of this family of compounds, possibly including those of bromantane.[6]
Examples
See also
External links
References
- ↑ Respiratory Infections | https://books.google.co.uk/books?id=qif7D7v2QgUC&pg=PA243&redir_esc=y#v=onepage&q&f=false
- ↑ Neurological Disorders: Course and Treatment | https://books.google.co.uk/books?id=q9h5Xg3pwAYC&pg=PA1047&redir_esc=y#v=onepage&q&f=false
- ↑ 3.0 3.1 Adamantane derivatives: Pharmacological and toxicological properties (review) | https://link.springer.com/article/10.1007%2FBF02524549
- ↑ Pharmacology of Actoprotectors: Practical Application for Improvement of Mental and Physical Performance" (PubMed.gov / NCBI) | https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3762282
- ↑ The synthesis and pharmacological evaluation of adamantane-derived indoles: cannabimimetic drugs of abuse. (PubMed.gov / NCBI) | https://www.ncbi.nlm.nih.gov/pubmed/23551277
- ↑ Involvement of the sigma 1 receptor in the modulation of dopaminergic transmission by amantadine. (PubMed.gov / NCBI) | https://www.ncbi.nlm.nih.gov/pubmed/15090047