Lysergamides
 |
This article is a stub. As such, it may contain incomplete or wrong information. You can help by expanding it. |
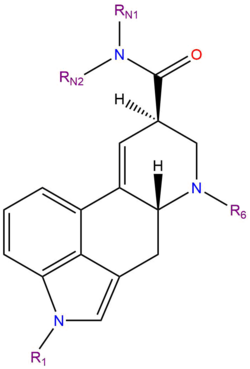
Lysergamides are amides of lysergic acid.
Ergoline refers to a class of compounds derived from alkaloids of a group of fungi known as ergot in the claviceps genus. These compounds typically have have strong psychedelic effects.
Chemistry
Lysergamides are polycyclic amides which have both phenethylamine and tryptamine groups embedded within their structure and a carboxamide group attached to carbon number eight. Varying the substituent attached to the nitrogen atoms has produced a variety of drugs with psychedelic effects as well as prescription drugs for treating headaches and inducing labor. Hydroxylation of the aromatic ring is one method of metabolizing lysergamides in humans and produces compounds with greater affinity to dopamine receptors.
Pharmacology
The psychedelic effects of lysergamides are believed to come from its efficacy at the 5-HT2A receptor as a partial agonist. Lysergamides are known to have affinity for a much greater variety of receptors than other psychedelic drugs.
However, the role of these interactions and how they result in the psychedelic experience continues to remain elusive.
List of substituted lysergamides
| Compound | RN1 | RN2 | R1 | R6 | Structure |
|---|---|---|---|---|---|
| 1P-ETH-LAD | CH2CH3 | CH2CH3 | C=OCH2CH3 | CH2CH3 | 
|
| 1P-LSD | CH2CH3 | CH2CH3 | C=OCH2CH3 | CH3 | 
|
| ALD-52 | CH2CH3 | CH2CH3 | C=OCH3 | CH3 | 
|
| AL-LAD | CH2CH3 | CH2CH3 | H | CH2CH=CH2 | 
|
| ETH-LAD | CH2CH3 | CH2CH3 | H | CH2CH3 | 
|
| LSA | H | H | H | CH3 | 
|
| LSD | CH2CH3 | CH2CH3 | H | CH3 | 
|
| LSH | CH(OH)CH3 | H | H | CH3 | 
|
| LSM-775 | CH2CH2O- | CH2CH2- | H | CH3 | 
|
| LSZ | CH(CH3)CH2- | CH(CH3)CH2- | H | CH3 | 
|
| PARGY-LAD | CH2CH3 | CH2CH3 | H | CH2C≡CH | 170px |
| PRO-LAD | CH2CH3 | CH2CH3 | H | CH2CH2CH3 | 
|
See also
References
 |
This article does not cite enough references. You can help by adding some. |