This is an unofficial archive of PsychonautWiki as of 2025-08-11T15:14:44Z. Content on this page may be outdated, incomplete, or inaccurate. Please refer to the original page for the most up-to-date information.
Category:Tryptamine
Tryptamine is a mono-amine alkaloid found in animals, plants and fungi.
Substituted tryptamine refers to a class of compounds which typically produce strong psychedelic effects.
Chemistry
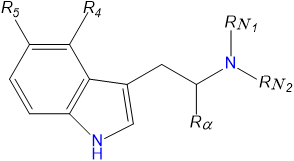
Tryptamine comprises an indole ring attached to a mono-amine chain. Hydrogen atoms around the structure can be substituted for other functional groups to produce drugs of varying potency, efficacy and half-life.
| Short Name | Origin | Rα | R4 | R5 | RN1 | RN2 | Full Name |
|---|---|---|---|---|---|---|---|
| Tryptamine | Natural | H | H | H | H | H | 3-(2-aminoethyl)indole / 2-(1H-indol-3-yl)ethanamine |
| Bufotenin | Natural | H | H | OH | CH3 | CH3 | 5-hydroxy-N,N-dimethyltryptamine |
| Nω-methylserotonin (norbufotenin) | Natural | H | H | OH | CH3 | H | 5-hydroxy-N-methyltryptamine |
| Serotonin | Natural | H | H | OH | H | H | 5-hydroxytryptamine |
| DMT | Natural | H | H | H | CH3 | CH3 | N,N-dimethyltryptamine |
| Melatonin | Natural | H | H | OCH3 | O=C-CH3 | H | 5-methoxy-N-acetyltryptamine |
| 5-Bromo-DMT | Natural | H | H | Br | CH3 | CH3 | 5-bromo-N,N-dimethyltryptamine |
| 5-MeO-DMT | Natural | H | H | OCH3 | CH3 | CH3 | 5-methoxy-N,N-dimethyltryptamine |
| 5-MeO-NMT | Natural | H | H | OCH3 | CH3 | H | 5-methoxy-N-methyltryptamine |
| NMT | Natural | H | H | H | H | CH3 | N-methyltryptamine |
| Norbaeocystin | Natural | H | OPO3H2 | H | H | H | 4-phosphoryloxy-tryptamine |
| Baeocystin | Natural | H | OPO3H2 | H | CH3 | H | 4-phosphoryloxy-N-methyl-tryptamine |
| Psilocybin | Natural | H | PO4 | H | CH3 | CH3 | 4-phosphoryloxy-N,N-dimethyltryptamine |
| Psilocin | Natural | H | OH | H | CH3 | CH3 | 4-hydroxy-N,N-dimethyltryptamine |
| Tryptophan | Natural | COOH | H | H | H | H | α-carboxyltryptamine |
| αET | Artificial | CH2CH3 | H | H | H | H | α-ethyltryptamine |
| αMT | Artificial | CH3 | H | H | H | H | α-methyltryptamine |
| DALT | Artificial | H | H | H | H2C=CH-CH2 | H2C=CH-CH2 | N,N-diallyltryptamine |
| DET | Artificial | H | H | H | CH2CH3 | CH2CH3 | N,N-diethyltryptamine |
| DiPT | Artificial | H | H | H | CH(CH3)2 | CH(CH3)2 | N,N-diisopropyltryptamine |
| DPT | Artificial | H | H | H | CH2CH2CH3 | CH2CH2CH3 | N,N-dipropyltryptamine |
| 5-MeO-αMT | Artificial | CH3 | H | OCH3 | H | H | 5-methoxy-α-methyltryptamine |
| 5-MeO-DALT | Artificial | H | H | OCH3 | H2C=CH-CH2 | H2C=CH-CH2 | 5-methoxy-N,N-diallyltryptamine |
| 4-HO-DET | Artificial | H | OH | H | CH2CH3 | CH2CH3 | 4-hydroxy-N,N-diethyltryptamine |
| 4-AcO-DMT | Artificial | H | OCOCH3 | H | CH3 | CH3 | 4-acetoxy-N,N-dimethyltryptamine |
| 4-HO-MET | Artificial | H | OH | H | CH3 | CH2CH3 | 4-hydroxy-N-methyl-N-ethyltryptamine |
| 4-HO-DIPT | Artificial | H | OH | H | CH(CH3)2 | CH(CH3)2 | 4-hydroxy-N,N-diisopropyltryptamine |
| 5-MeO-DIPT | Artificial | H | H | OCH3 | CH(CH3)2 | CH(CH3)2 | 5-methoxy-N,N-diisopropyltryptamine |
| 4-HO-MiPT | Artificial | H | OH | H | CH(CH3)2 | CH3 | 4-hydroxy-N-isopropyl-N-methyltryptamine |
| Sumatriptan | Artificial | H | H | CH2SO2NHCH3 | CH3 | CH3 | 5-(methylaminosulfonylmethylene)-N,N-dimethyltryptamine |
| Zolmitriptan | Artificial | H | H | -(CHNHC=OOCH2) | CH3 | CH3 | 5-( 4-(S)-1,3-oxazolidin-2-one)-N,N-dimethyltryptamine |
| Short Name | Origin | Rα | R4 | R5 | RN1 | RN2 | Full Name |
Pharmacology
Tryptamines are believed to produce their psychedelic effect primarily by partial agonism of 5-HT2A receptors.
Several neurotransmitters are derived from tryptamine, such as:
References
Pages in category "Tryptamine"
The following 36 pages are in this category, out of 36 total.